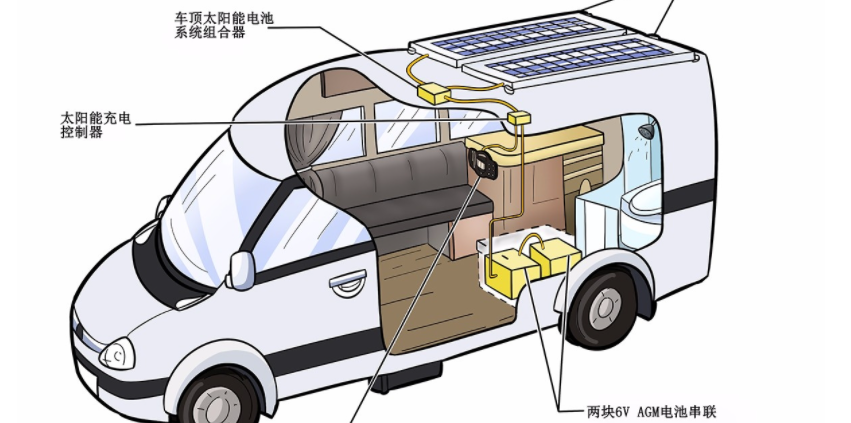
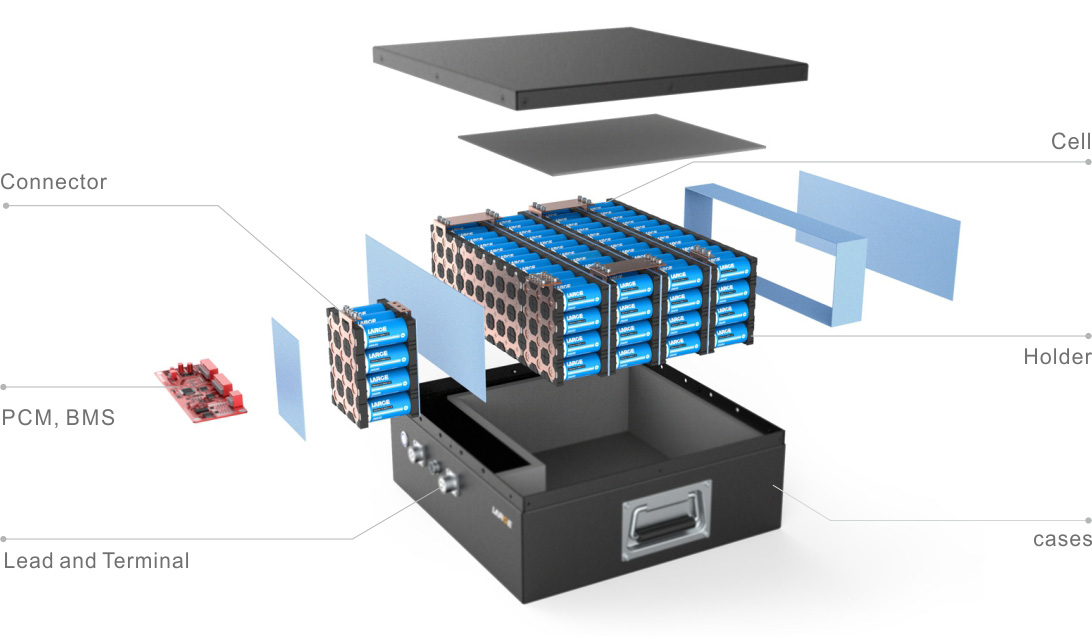
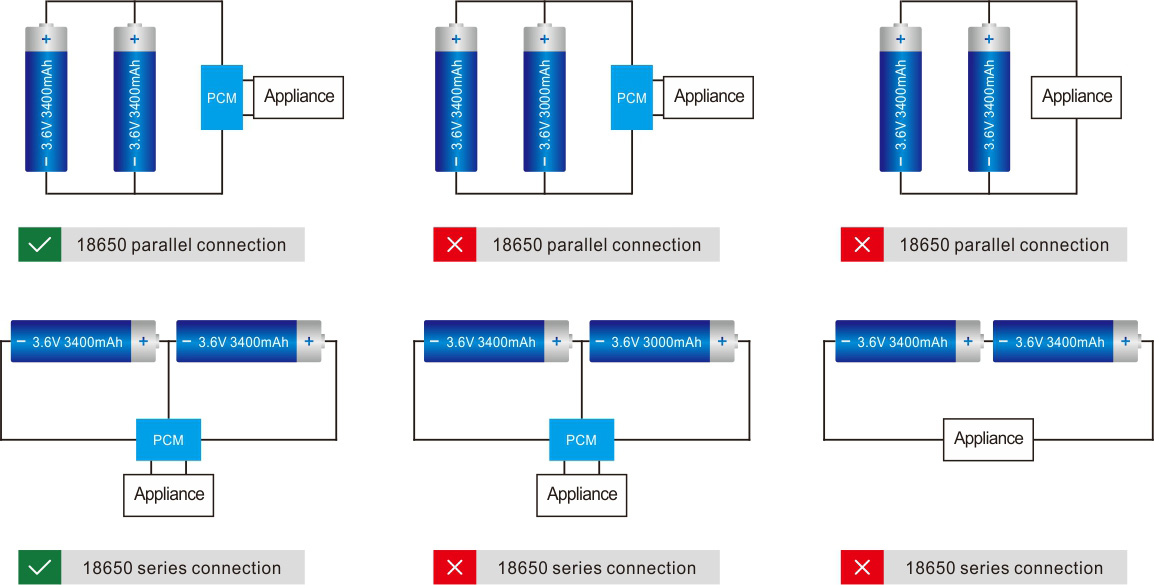
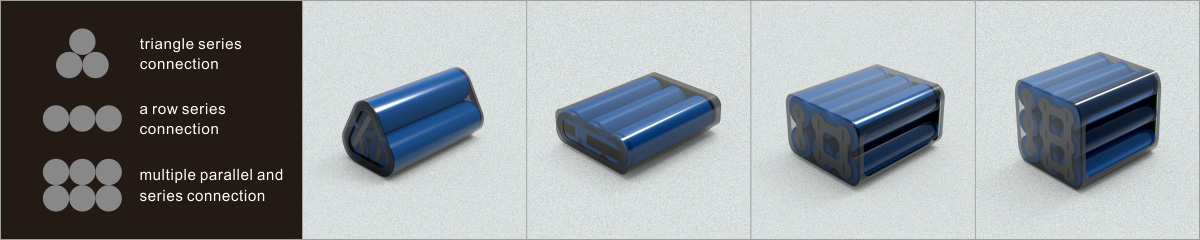
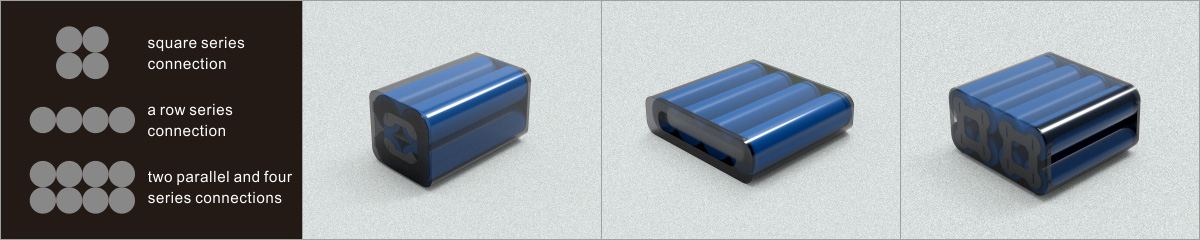
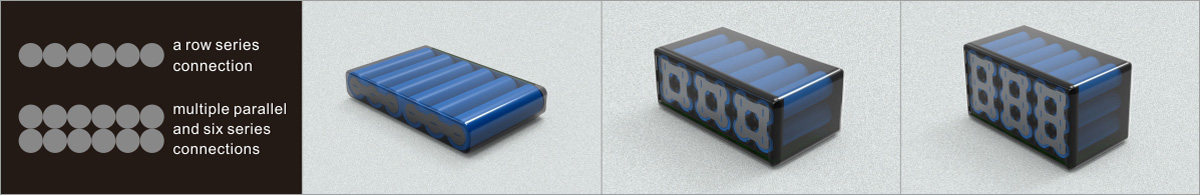
Learn about unique applications and what features to look for when choosing a battery.
Consumers are the first to hear about an apparent battery breakthrough. To get top media attention, the new super battery promises to also satisfy the need for electric vehicle (EV). Personal mobility is an emotional issue that cannot be suppressed, even if it harms the environment. The industrial space, on the other hand, is more conservative and it appears to lag behind. Not so. Industry is rational and understands the many constraints of the battery by focusing on reliability, economy, longevity and safety.
Batteries for Traction
Wheelchairs, scooters and golf cars mostly use lead acid batteries. Even though heavy, lead acid works reasonably well and only moderate attempts are made to switch to other systems Li-ion will be a natural alternative in many applications.
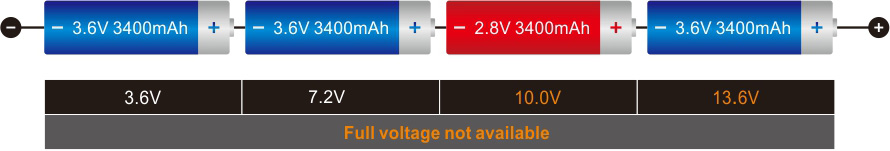
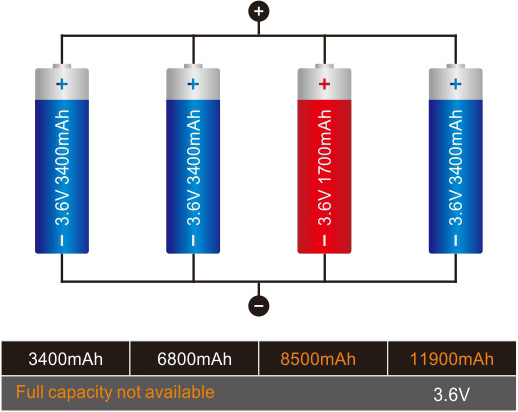
Although Li-ion is more expensive than lead acid, the cycle cost can be lower because of the longer life. A further advantage of Li-ion over lead- and nickel-based batteries is the low maintenance. Li-ion can be left at any state-of-charge without adverse side effects. In contrast, NiCd and NiMH need an occasional full discharge to prevent memory and lead acid requires a saturated charge to prevent sulfation.
Most wheelchairs and golf cars are still powered with lead acid, so are forklifts. With forklifts, the heavy weight is less of an issue, but the long charging time is a disadvantage for warehouses operating 24 hours a day. Some forklifts are fitted with fuel cells that charge the battery while the vehicle is in use. The battery can be made smaller but not eliminated because the fuel cell has poor power delivery and has a sluggish ramp-up on demand; the battery remains the primary power source.
The heavier the wheeled application is, the less suitable the battery becomes. This does not prevent engineers from looking into large battery systems to replace the polluting internal combustion engine (ICE). One such application is the Automatic Guided Vehicle (AGV) system at ship ports. AGVs run 24 hours a day and the vehicles cannot be tied up for lengthy charging intervals. Li-ion solves this in part by replacing the very large 10-ton, 300kWh lead acid with a battery that is lighter and can be charged more quickly. But very large batteries have a limitation because of weight, charging time and infrastructure and the fuel cell may solve large traction systems as described in BU-1005 if burning fossil fuel is not an option.
However, no economical battery solution exists yet for large traction systems and burning fossil fuel cannot be fully avoided. While a modern Li-ion battery delivers about 150Wh/kg of energy, the net calorific value (NCV) of fossil fuel is over 12,000Wh/kg. Even at the low 25-percent efficiency of an ICE engine, the energy from a battery is fractional compared to fossil fuel (see BU-1007: Net Calorific Value). Furthermore, the ICE can operate in extreme cold and heat, a task the battery struggles to meet.

Batteries for Aviation
The duty of batteries on board an aircraft is to feed navigation and emergency systems when the Auxiliary Power Unit (APU) is off or during an emergency in flight. The battery provides power for braking, ground operation and starting the APU. In the event of engine failure, the batteries must supply energy from 30 minutes to 3 hours. Each aircraft must also have enough battery power to facilitate a safe landing. During flight, the electrical power is supplied by generators and, similar to a car, the on-board battery could be disconnected if so required.
Most commercial jetliners use flooded nickel-cadmium. Starting a large aircraft begins by spooling the APU, a small turbine engine located at the tail section of an airplane. This takes significantly longer and requires more energy than cranking a reciprocating engine of similar size. The spooling speed of the APU must be sufficiently high to attain compression for self-sustained ignition. Starting takes about 15 seconds and consumes 15kW of energy. Once running, an air compressor or hydraulic pump jumpstarts the large jet engines one-by-one.
Smaller aircraft often have sealed lead-acid. Although heavier than NiCd, lead acid requires less maintenance. The 12 and 24V aviation batteries are rated in IPP (current peak power)* and IPR (current power rating)** rather than CCA (cold cranking amps) as is common in the automotive industry. IPP and IPR are the International Electrotechnical Commission (IEC 60952-1) standard for aircraft batteries and FAA TSO-C173 that allow a battery to spool each engine for 25–40 seconds at high current.
Modern jet fighters spool the jet engines with Li-ion, so does the Boeing 787 Dreamliner. The Airbus 350 offers the option of either chemistry. As the on-board functions of an airliner move from hydraulic to electrical, larger batteries are required. The higher energy-dense Li-ion satisfies this demand better than NiCd and lead acid. However, unexpected Li-ion failure with serious consequences may move airplane makers back to NiCd. All batteries are subject to breakdowns; there are also reported heat failures with NiCd, but these can be better managed than Li-ion.
NiCd provides durability and reliable service, but it needs high maintenance that includes exercising the battery to eliminate memory. The service of the main-ship battery consists of a total discharge and shorting each cell for 24 hours with a strap. The battery is also checked for capacity with a battery analyzer. Smaller NiCd batteries have different service requirements.
Although aircraft carry many different batteries aboard, their sole purpose is to start the engine and provide backup power when the engines are off. Large aircraft will continue to fly on fossil fuel as batteries are not yet practical for propulsion. Small battery-powered airplanes are being tried for pilot training and to fly short hops but these are experimental only. Weight and reliability on an aging battery remain major concerns.
| *Ipp: |
Peak current delivered at 0.3 seconds into a 15 second controlled discharge at a constant terminal voltage of half the nominal battery voltage. |
| **Ipr: |
This is the discharge current at the conclusion of a 15 second controlled discharge at a constant terminal voltage of half the nominal battery voltage. |
Batteries for Aerospace
Early satellites used NiCd batteries, and this led to the discovery of the “memory” phenomenon. The battery followed a routine discharge schedule but when more energy was demanded, the battery could remember. The voltage would drop as if to protest against unwanted overtime.
NiCd was replaced by nickel-hydrogen as a battery with an exceptional long service life. Entrepreneurs tried to introduce this amazing battery for commercial use but high price and large size spoiled market acceptance. Each cell costs around $1,000 and has the appearance of a small steam engine with a steel pressure tank.
Li-ion is the battery of choice for satellites. It is light-weight, easy to charge, durable and cycles well. Li-ion can dwell in any SoC for an extended length of time without adverse side effects; it has low self-discharge and is virtually maintenance free.
The Mars Curiosity Rover uses specially designed lithium nickel oxide cells (LiNiCo) in 8S2P formation (eight cells in series and two in parallel) that is only partially charged and discharged to stretch longevity. Under this regime, the life span is four years and roughly 700 sol. (The term sol is used by planetary astronomers to refer to the duration of a solar day on Mars.) The 43Ah cells, of which two are in parallel, have a maximum discharge C-rate of 0.55C.
NASA wants Li-ion batteries to last for 7 years and 37,000 cycles with a DoD of 40 to 60 percent. NASA labs reveal that end-of-life is connected with the growth of the SEI layer on the anode, loss of cathode material, loss of conductive path, plating of metallic lithium and electrolyte oxidation. Large 140Ah Li-ion cells are in development that promise to last up to 18 years. (See also BU-808B: What causes Li-ion to die?)
Stationary Batteries
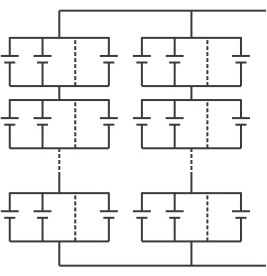
With a growing choice of batteries for energy storage systems (ESS), the selection should not be based on price alone. Cost per kWh says little without also examining the total cost of ownership that includes cost per cycle, longevity and eventual replacement.
Lead acid is well suited for duties that need only occasional discharges. The flow battery and sodium-sulfur battery work well for large systems requiring regimented discharges, while lithium ion is recommended for small to medium systems delivering short discharging with fast charging ability multiple times a day.
Traditionally, stationary batteries have been lead acid. Size and weight is of lesser concern, and the limited cycle count does not pose a problem when the batteries are seldom discharged. Large stationary batteries are mostly flooded and require regular checking of the electrolyte level. This maintenance can be reduced with an automatic watering system.
Valve-regulated lead acid (VRLA) is the low-maintenance version of the flooded lead acid. It is said that VRLA can be installed and forgotten, but this is often taken to the extreme in that the batteries get neglected. Maintenance includes checking the voltage, internal resistance and sometimes capacity levels.
Applications that are exposed to hot and cold temperatures as well as those requiring deep cycling are often served by flooded nickel-cadmium. These batteries are more rugged than lead acid but are roughly four times the cost. Flooded nickel-cadmium batteries are non-sintered and are less subject to memory that the sintered versions, which are sealed, but some maintenance is still required. NiCd is the only battery that can be rapidly charged with minimal stress.
Many stationary batteries are also served by Li-ion. Li-ion comes with many advantages, but the battery does not perform as well as NiCd and lead acid at low temperature. Another battery that is making a comeback for stationary use is nickel-iron.Inventor Thomas Edison promoted NiFe for the electric vehicle, but it eventually lost out to lead acid due to high cost and high self-discharge. Improvements have eliminated some of the failings, and the superior durability of this battery is gaining renewed interest.
Energy Storage Systems (Grid Storage Batteries)
Renewable energy sources such as wind and sun do not provide a steady stream of energy, nor do they always harmonize with user demand. Large energy storage systems (ESS) called load leveling or grid storage batteries are needed to provide a seamless service.
ESS enjoys a large growth trajectory to move from coal and oil to renewable resources. ESS installations in South Africa alone are estimated to reach 1,500MWh by 2021. Chemistries under consideration are flow batteries, Li-ion, lead acid and zinc-bromine. Zinc-bromine is a type of hybrid flow battery that can be regarded as an electroplating machine. During charge, zinc electroplates onto conductive electrodes forming bromine; the process reverses on discharge. Another leading ESS battery is the high temperature sodium-sulfur battery.
Storing energy to supply peak shaving power is not new. Hydroelectric power stations use excess electricity to pump water back up to the reservoir at night for use the next day. With an efficiency factor of 70–85 percent, pumped hydro is easier to manage than adjusting the generators to the exact power need. Pumping compressed air into large underground cavities and underwater balloons are also being used to store energy.
Flywheels also serve as energy storage. Large electric motors rev up one-ton flywheels when excess energy is available to supply brief energy deficiencies. High-speed flywheels spin at over 30,000 rpm on magnetic bearings in a vacuum chamber. Electric motors/generators with permanent magnets charge and discharge the kinetic energy on demand.
Modern flywheels replace steel with carbon fibers to withstand higher rotations of up to 60,000 rpm. Energy increases by the square of speed, providing four times the power at a reduced weight. Should the flywheel fail, the housing prevents shrapnel form escaping.
Using flywheels to store kinetic energy is not new. In the 1940s and 1950s, city busses in Switzerland were powered by flywheels. An electric motor would spin a 3-ton flywheel to 3,000 rpm in 3 minutes. Turning into a generator, the motor would then transform the energy back into electricity. Each charge would yield for 6km (3.75mi) on a flat road. The bus was pollution-free but the gyroscope action resisted changing direction on a windy road.
Load leveling is gravitating towards Li-ion because of small footprint, low maintenance and long life. Li-ion does not suffer from sulfation as lead acid does when not fully charged periodically. This can be a major drawback with installations when demand exceeds supply. Li-ion also has the benefit of being light-weight and semi-portable for installations in remote locations. The negatives of Li-ion are its high price and low performance at cold temperature. A further drawback is the inability to charge below freezing.
Li-ion has come down in price and Table 1 provides a cost comparison with lead acid for grid storage applications. Although the initial price of Li-ion is higher than lead acid, the cost per cycle is lower in deep-cycle applications. Li-ion is said to gain in market share but lead acid will keep its stronghold.
|
LEAD ACID |
LI-ION |
| Battery cost |
$20,000 |
$52,000 |
| Lifespan |
500 cycles at 50% DoD |
1,900 cycles at 90% DoD |
| Cost per cycle |
$40 |
$28 |
Table 1: Cost comparison of lead acid and Li-ion for renewable energy. Li-ion has a higher initial cost but is lower on the cost per cycle. Prices are estimated.
Courtesy: http://www.powertechsystems.eu/en/technics/lithium-ion-vs-lead-acid-cost-analysis
The energy output of a large industrial wind turbine is 1 megawatt (MW) and more; the biggest units have grown to 10MW. Several turbines form a wind farm that produces 30–300MW. To fathom a megawatt, 1MW feeds 50 houses or a Walmart superstore.
Not all renewable energy systems include load leveling batteries. The batteries simply get too large and the investment cannot always be justified. If supported by batteries, a 30MW wind farm uses a storage battery of about 15MW. This is the equivalent of 20,000 starter batteries or 176 Tesla S 85 EVs with an 85kWh battery each. The cost to store energy in a battery is high, and some say it doubles the cost to a direct supply.
The battery management system (BMS) keeps the battery at about 50 percent charge to allow absorbing energy on wind gusts and delivering on high load demands. Modern BMS can switch from charge to discharge in less than a second. This helps stabilize the voltage on transmission lines, also known as frequency regulation.