What Is C-rate?
Observe how the charge and discharge rates are scaled and why it matters.
Charge and discharge rates of a battery are governed by C-rates. The capacity of a battery is commonly rated at 1C, meaning that a fully charged battery rated at 1Ah should provide 1A for one hour. The same battery discharging at 0.5C should provide 500mA for two hours, and at 2C it delivers 2A for 30 minutes. Losses at fast discharges reduce the discharge time and these losses also affect charge times.
A C-rate of 1C is also known as a one-hour discharge; 0.5C or C/2 is a two-hour discharge and 0.2C or C/5 is a 5-hour discharge. Some high-performance batteries can be charged and discharged above 1C with moderate stress. Table 1 illustrates typical times at various C-rates.
| C-rate | Time | Table 1: C-rate and service times when charging and discharging batteries of 1Ah (1,000mAh)
|
|
| 5C | 12 min | ||
| 2C | 30 min | ||
| 1C | 1h | ||
| 0.5C or C/2 | 2h | ||
| 0.2C or C/5 | 5h | ||
| 0.1C or C/10 | 10h | ||
| 0.05C or C/20 | 20h |
The battery capacity, or the amount of energy a battery can hold, can be measured with a battery analyzer. The analyzer discharges the battery at a calibrated current while measuring the time until the end-of-discharge voltage is reached. For lead acid, the end-of-discharge is typically 1.75V/cell, for NiCd/NiMH 1.0V/cell and for Li-ion 3.0V/cell. If a 1Ah battery provides 1A for one hour, an analyzer displaying the results in percentage of the nominal rating will show 100 percent. If the discharge lasts 30 minutes before reaching the end-of-discharge cut-off voltage, then the battery has a capacity of 50 percent. A new battery is sometimes overrated and can produce more than 100 percent capacity; others are underrated and never reach 100 percent, even after priming.
When discharging a battery with a battery analyzer capable of applying different C rates, a higher C rate will produce a lower capacity reading and vice versa. By discharging the 1Ah battery at the faster 2C-rate, or 2A, the battery should ideally deliver the full capacity in 30 minutes. The sum should be the same since the identical amount of energy is dispensed over a shorter time. In reality, internal losses turn some of the energy into heat and lower the resulting capacity to about 95 percent or less. Discharging the same battery at 0.5C, or 500mA over 2 hours, will likely increase the capacity to above 100 percent.
To obtain a reasonably good capacity reading, manufacturers commonly rate alkaline and lead acid batteries at a very low 0.05C, or a 20-hour discharge. Even at this slow discharge rate, lead acid seldom attains a 100 percent capacity as the batteries are overrated. Manufacturers provide capacity offsets to adjust for the discrepancies if discharged at a higher C rate than specified. Figure 2 illustrates the discharge times of a lead acid battery at various loads expressed in C-rate.
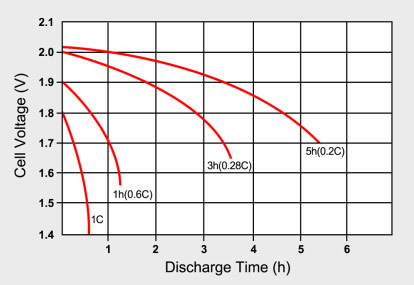 |
| Figure 2: Typical discharge curves of lead acid as a function of C-rate. Smaller batteries are rated at a 1C discharge rate. Due to sluggish behavior, lead acid is rated at 0.2C (5h) and 0.05C (20h). |
While lead- and nickel-based batteries can be discharged at a high rate, the protection circuit prevents the Li-ion Energy Cell from discharging above 1C. The Power Cell with nickel, manganese and/or phosphate active material can tolerate discharge rates of up to 10C and the current threshold is set higher accordingly.


