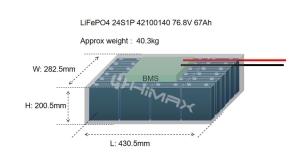
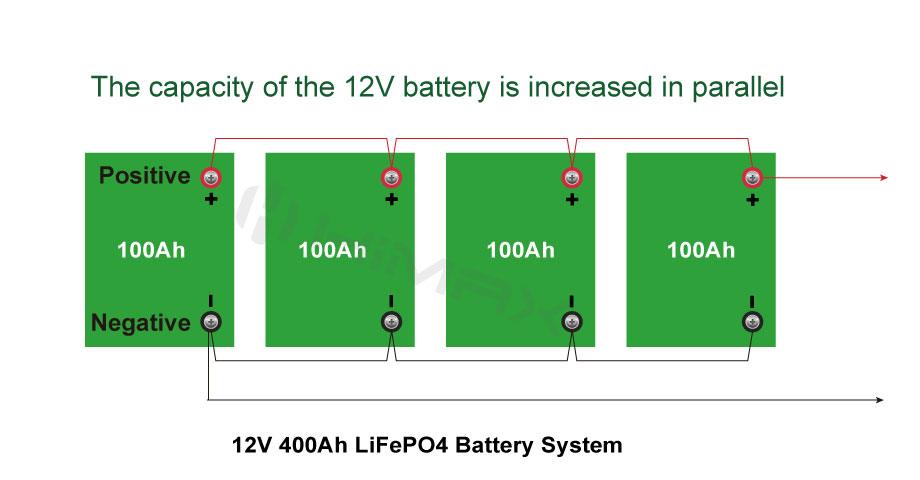
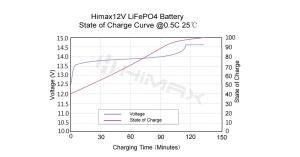
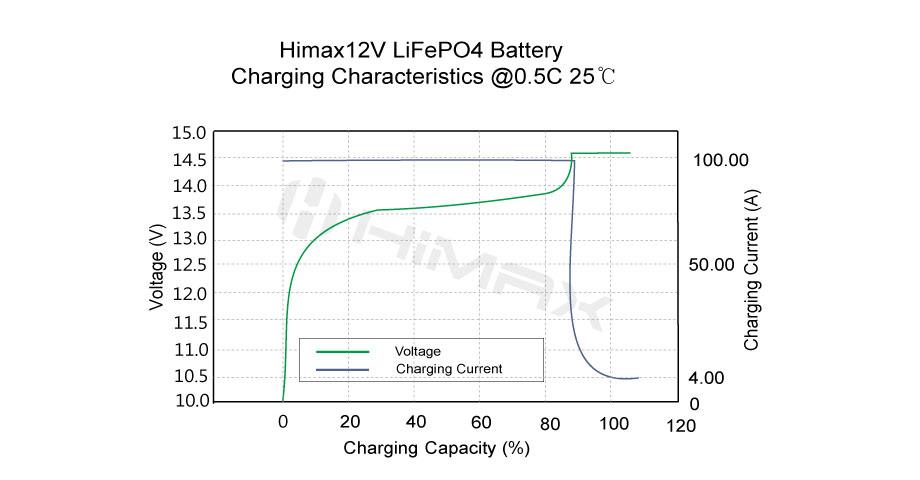
In the landscape of modern battery technologies, LiFePO4 (lithium iron phosphate) batteries have emerged as a standout choice due to their reputed safety and reliability. Especially prevalent in applications where safety cannot be compromised, such as electric vehicles and renewable energy systems, LiFePO4 batteries offer an appealing alternative to traditional lithium-ion batteries. This article delves deeply into the safety features of LiFePO4 batteries, compares them with other battery types, and discusses how Himax Electronics utilizes this technology to deliver superior safety in battery solutions.

Understanding LiFePO4 Battery Safety
Chemical and Thermal Stability:
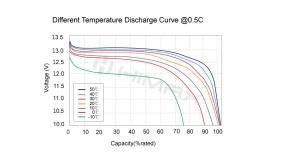
LiFePO4 batteries are constructed using lithium iron phosphate as the cathode material, which inherently provides significant safety advantages. The phosphate chemistry grants these batteries a strong bond that withstands extreme abuse conditions better than other lithium chemistries. This chemical stability leads to superior thermal stability, meaning LiFePO4 batteries are less likely to suffer from thermal runaway—a condition where increased temperature causes a reaction that continuously increases temperature, leading to fires or explosions.
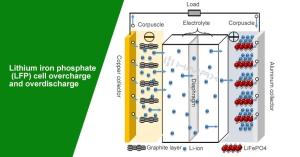
Overcharge and Overdischarge Resistance:
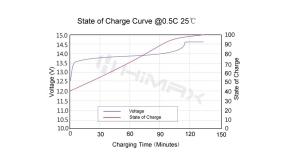
One of the significant risks with battery technologies is the potential for overcharging or deep discharging, which can compromise battery integrity and safety. LiFePO4 batteries inherently resist both conditions. They maintain a stable voltage range during charging and discharging, which helps prevent the scenarios where voltage spikes or dips could lead to hazardous situations.
Comparative Safety with Other Battery Technologies
LiFePO4 vs. Lithium-Cobalt Oxide (LiCoO2):
While LiCoO2 batteries, commonly used in mobile devices and laptops, store higher amounts of energy, they pose higher risks of thermal runaway and are more sensitive to high temperatures. In contrast, LiFePO4 batteries operate safely at higher temperatures and are significantly less prone to catastrophic failure when damaged.
LiFePO4 vs. Nickel-Metal Hydride (NiMH):
NiMH batteries, found in many hybrid vehicles, are less volatile than traditional lithium-ion batteries but still lag behind LiFePO4 in terms of overall safety, lifespan, and weight efficiency. LiFePO4 batteries offer a lighter, more efficient solution with a considerably longer life cycle and better stability during thermal stress.
Real-World Safety Applications of LiFePO4 Batteries
Electric Vehicles (EVs):
Safety is paramount in EVs due to the large amount of energy stored and utilized. LiFePO4 batteries are favored in this application because of their resistance to high temperatures and their stability in the event of an accident. This safety profile significantly reduces the risk of fires and explosions in crashes, making EVs safer for consumers.
Solar Energy Systems:
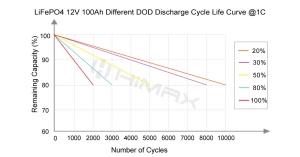
LiFePO4 batteries are ideal for solar energy storage due to their long lifespan and the ability to deep cycle without significant degradation. Homeowners and businesses using solar panels benefit from the peace of mind that comes with installing LiFePO4 batteries, which are not prone to catching fire even when subjected to high temperatures or overcharging conditions typical in solar energy applications.
Addressing Safety Concerns with Proper Handling and Maintenance
Despite their inherent safety, the performance and longevity of LiFePO4 batteries can be optimized through proper handling and maintenance:
- Appropriate Charging Practices: Using a charger specifically designed for LiFePO4 chemistry is crucial, as it ensures the battery is charged within its safe voltage range.
- Regular Inspections: Periodic checks for damage or wear can help prevent potential safety issues, especially in systems where batteries are exposed to environmental stressors.
- Installation Considerations: Correct installation in a battery management system can monitor the battery’s health, providing alerts for potential issues and ensuring the battery operates within safe parameters.
Himax Electronics: Enhancing Safety with LiFePO4 Batteries
At Himax Electronics, we understand the importance of safety in battery technology. Our LiFePO4 batteries are designed not only to meet but exceed safety standards. We offer customized solutions that integrate advanced battery management systems to enhance safety features further and ensure that our batteries deliver optimal performance and reliability in any application. Our commitment to quality and safety in our LiFePO4 offerings allows us to provide our customers with reliable, efficient, and safe energy solutions.
Conclusion
LiFePO4 batteries represent a significant advancement in battery technology, offering enhanced safety without compromising on performance or efficiency. Whether for personal electronics, electric vehicles, or large-scale energy storage, LiFePO4 batteries provide a reliable and safe alternative to traditional battery chemistries. At Himax Electronics, we are dedicated to leveraging