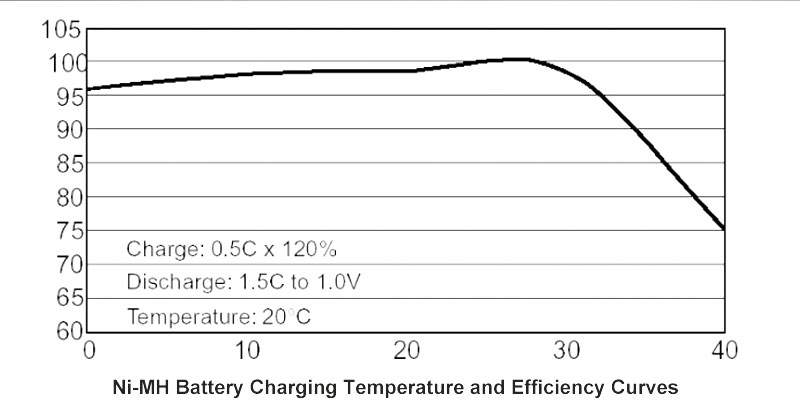
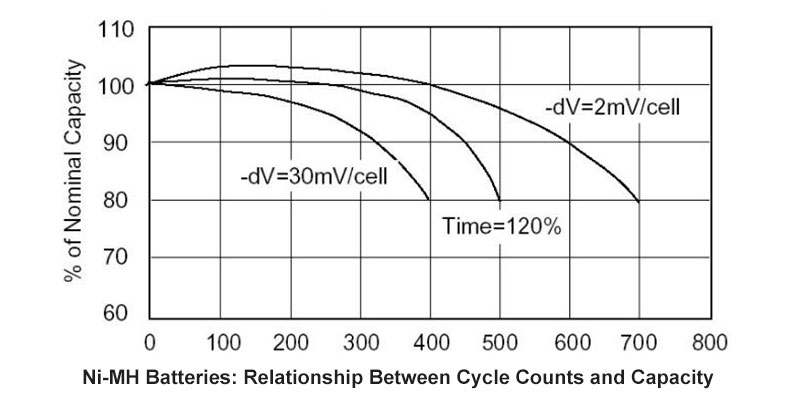
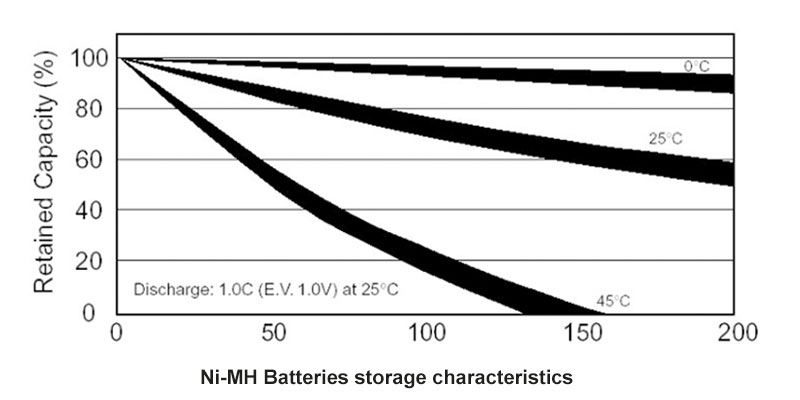
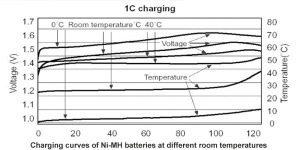
In today’s world, the demand for reliable and sustainable energy solutions is greater than ever. Among the many battery technologies available, Nickel-Metal Hydride (NiMH) batteries stand out for their unique advantages. These batteries are not only eco-friendly but also provide the reliability and performance required in a wide range of applications—from electric tools to medical devices, and smart home systems. At Himax Battery, we specialize in designing and manufacturing high-quality NiMH battery packs tailored to meet the specific needs of our customers, all at a competitive price.
Here’s a closer look at why NiMH battery packs are essential for various industries and how Himax can help you benefit from their superior features.
The Irreplaceable Advantages of NiMH Battery Packs
NiMH batteries are highly versatile and offer performance that other battery technologies can’t always match. They’re perfect for high-power applications, where reliability, safety, and long battery life are critical.
1. High Energy Density and Reliable Performance
NiMH batteries have a high energy density, meaning they can store a large amount of energy for their size, making them ideal for power-intensive devices. Whether it’s an electric power tool or a medical device, these batteries offer a long-lasting, reliable power source. For industries where performance is paramount, such as healthcare and emergency backup systems, NiMH batteries provide the necessary energy to keep devices operational in critical situations.

2. Eco-Friendly and Safe
Himax’s NiMH battery packs are safe to use and environmentally friendly. Unlike traditional nickel-cadmium (NiCd) batteries, NiMH batteries are free from toxic heavy metals like cadmium, making them a greener, more sustainable energy choice. For businesses looking to reduce their environmental impact while maintaining high standards of quality, NiMH batteries are the ideal solution. Our batteries are also CE and RoHS certified, ensuring they meet international safety and environmental standards.
3. Long Lifecycle and Cost-Efficiency
One of the standout features of NiMH batteries is their long lifecycle. These batteries are built to withstand many charge and discharge cycles without significant performance degradation. For businesses, this translates into reduced replacement costs and more reliable products over time. Plus, as a battery manufacturer, Himax provides direct-to-customer pricing, meaning you get high-quality, custom-designed battery packs at a lower cost compared to buying from resellers or distributors.
Key Applications for NiMH Battery Packs
NiMH batteries have become the go-to power source for various industries. Let’s explore the specific applications where these batteries shine:
1. Electric Tools and Industrial Equipment
Electric tools such as drills, saws, and lawnmowers require batteries that can deliver high discharge rates and durable performance. NiMH batteries are perfect for these tools as they provide consistent power and longevity, ensuring they can handle demanding tasks without faltering. NiMH’s thermal stability and resistance to wear make them the go-to battery for power tools in construction and industrial settings.
2. Medical Devices
In the medical field, the reliability of power sources can be a matter of life and death. Himax’s NiMH battery packs power portable medical equipment, including infusion pumps, portable ECG machines, and patient monitors. These batteries offer a safe and consistent power supply, ensuring critical medical devices continue to function in emergency and mobile environments. Their rechargeable nature and environmentally friendly properties also make them an excellent choice for healthcare providers aiming to reduce waste.
3. Backup Power Systems
Uninterrupted power supply (UPS) systems rely on batteries that can handle long discharge cycles and provide consistent voltage output. NiMH batteries are an ideal solution for backup power in systems such as home security alarms, emergency lighting, and data centers. They maintain high performance over time, ensuring that the backup system kicks in whenever the main power source fails.
4. Smart Homes and Consumer Electronics
The rise of smart home systems means there is an increasing need for reliable, low-maintenance batteries. NiMH batteries are used in a variety of smart home devices like thermostats, security cameras, and motion detectors. These devices require long-lasting batteries that perform well in continuous charge/discharge cycles—a feature NiMH batteries offer in abundance.
Why Choose Himax Battery for Your NiMH Solutions?
At Himax Battery, we not only supply high-quality NiMH battery packs, but we also provide tailored solutions that meet the specific needs of our customers. Here’s how we stand out in the competitive battery market:
1. Custom Battery Solutions (OEM/ODM Services)
Every application is different, and we understand that your battery needs might require custom specifications. Whether you need a specific voltage, capacity, or battery form factor, Himax offers OEM/ODM services to design and manufacture battery packs that perfectly align with your product requirements. Our experienced engineers work directly with you to deliver optimized solutions that fit your technical needs.

2. Direct Factory Pricing
As a battery manufacturer, Himax cuts out the middleman, allowing us to offer direct pricing to our clients. This means you can get the highest-quality NiMH batteries at the best possible price, helping you save costs while maintaining top-notch performance in your products.
3. Comprehensive Support
From pre-sales consultation to after-sales service, Himax is committed to providing support every step of the way. Our team of experts ensures that you receive the right battery solution for your application and offers ongoing support for installation, usage, and maintenance.
Conclusion: Powering the Future with Himax NiMH Batteries
NiMH battery packs are crucial for a range of industries, offering superior performance, safety, and environmental benefits. At Himax Battery, we pride ourselves on delivering high-quality, customizable NiMH battery solutions designed to meet the unique needs of your business. Whether you are looking for reliable power for medical devices, industrial equipment, or smart home systems, Himax is here to provide the best battery technology at competitive prices.
Contact us today to learn more about how our NiMH battery packs can power your business with reliability, cost-effectiveness, and sustainability.