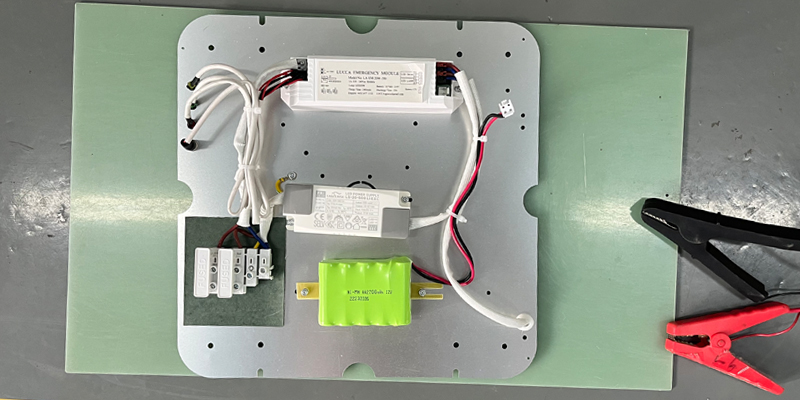
What Is a 4.8V 220mAh 2/3 AAA NiMH Battery?
Definition and Structure
The 4.8V 220mAh 2/3 AAA NiMH battery is a rechargeable battery that uses nickel-metal hydride (NiMH) technology. Its core components include a positive electrode made of nickel hydroxide, a negative electrode made of a hydrogen-absorbing alloy, and an electrolyte typically composed of potassium hydroxide solution. This battery is made up of four 1.2V cells connected in series, resulting in a total voltage of 4.8V and a capacity of 220mAh. The “2/3 AAA” refers to its size—smaller than a standard AAA battery (which measures 10.5mm in diameter and 44.5mm in length), with a length of about 30mm. This compact size makes it perfect for devices with limited space.
Technical Features
NiMH batteries are known for their high energy density, offering 2-3 times more capacity than traditional nickel-cadmium (NiCd) batteries, which means they can store more power in a smaller package. They’re also more eco-friendly since they don’t contain harmful substances like cadmium, aligning with modern green energy trends. Plus, NiMH batteries can be recharged hundreds of times, making them ideal for devices that require frequent charging cycles.
Why It’s Suitable for Security Alarm Systems
Security alarm systems often need batteries that are small, have long standby times, and deliver stable performance. The 4.8V 220mAh 2/3 AAA NiMH battery’s compact size allows it to fit easily into small alarm devices like door/window sensors or infrared detectors. Its low self-discharge feature ensures the device retains enough power during extended standby periods, preventing failures due to low battery levels. These qualities make this battery an excellent choice for security alarm systems.
Performance Advantages of the 4.8V 220mAh 2/3 AAA NiMH Battery in Security Alarm Systems
The 4.8V 220mAh 2/3 AAA NiMH battery stands out as a top power source for security alarm systems due to its impressive performance. These systems demand batteries with high stability, long-lasting power, and eco-friendliness, and this NiMH battery meets those needs with its unique technical advantages. Below, we’ll dive into the five key benefits of using this battery in security alarm systems to help you understand its value.
Advantage 1: Low Self-Discharge Rate for Long-Term Standby
Security alarm systems often need to stay on standby for long periods, so a battery’s self-discharge rate directly impacts its reliability. Standard NiMH batteries have a self-discharge rate of about 1%-5% per day, but advanced low self-discharge NiMH batteries can retain 70%-85% of their charge after a year in storage. This low self-discharge feature ensures that security alarm systems remain ready to operate even when not frequently used, preventing device failures due to power loss. For security equipment that needs to run reliably over the long term, this low self-discharge rate is a game-changer, boosting both dependability and user experience.
Advantage 2: High Stability and Safety
NiMH batteries are chemically stable and can operate effectively across a wide range of temperatures, typically from -20°C to 60°C. Security alarm systems may be installed in various environments, such as freezing winters or scorching summers, and the battery needs to handle these extremes. The 4.8V 220mAh 2/3 AAA NiMH battery has been rigorously tested to ensure stable power delivery in all conditions, avoiding performance drops due to temperature changes. Additionally, NiMH batteries have no risk of explosion or leakage, offering high safety and providing a dependable power source for security alarm systems.
Advantage 3: Compact Size and Compatibility
Security alarm systems are often designed to be compact, especially devices like door/window sensors and infrared detectors, which have strict size requirements for batteries. The 2/3 AAA size (10.5mm in diameter, about 30mm in length) is smaller than a standard AAA battery, making it a perfect fit for these devices’ battery compartments. Plus, the 4.8V voltage matches the power needs of most security alarm systems, so no additional circuitry adjustments are needed. This battery’s excellent size and voltage compatibility offer greater flexibility in device design, meeting the space constraints of small security equipment.
Advantage 4: Long Cycle Life to Reduce Maintenance Costs
For business users, maintenance costs are a big factor when choosing batteries. The 4.8V 220mAh 2/3 AAA NiMH battery has a cycle life of 500-1000 charges, meaning it can be recharged and used for years under normal conditions without needing frequent replacements. Compared to disposable batteries or those with shorter lifespans, this long cycle life significantly cuts down on maintenance and replacement costs for businesses. With optimized internal materials and manufacturing processes, this battery’s durability is further enhanced, delivering greater cost savings over time.
Advantage 5: Eco-Friendly and Compliant
Sustainability is a key consideration for modern businesses. NiMH batteries contain no harmful heavy metals like cadmium and comply with international environmental regulations, such as the EU Battery Directive (Directive 2006/66/EC). The 4.8V 220mAh 2/3 AAA NiMH battery has passed multiple environmental certifications, meeting the needs of companies focused on sustainable practices. Choosing this battery not only improves device performance but also reflects a commitment to environmental responsibility, contributing to green production and sustainability.
How to Choose the Right NiMH Battery for Security Alarm Systems
Voltage and Capacity
When selecting a battery for a security alarm system, the first step is to ensure its voltage and capacity meet the device’s requirements. The 4.8V 220mAh 2/3 AAA NiMH battery is designed to work with most small alarm devices, but it’s a good idea to double-check your equipment’s specifications before purchasing to confirm compatibility.
Size Compatibility
The 2/3 AAA size is ideal for small devices, but battery compartment designs can vary. Before buying, measure your device’s battery compartment to ensure the battery will fit properly.
Charging Tips
To extend battery life, use a smart charger to avoid overcharging or over-discharging. A charging current of C/10 (22mA) is recommended to prevent overheating and maintain optimal performance during charging.
Purchasing Advice
Business users should focus on brand reliability, bulk discounts, and after-sales support when buying batteries. Opting for a brand with a strong track record ensures product quality, while bulk discounts and solid support can add value to your purchase.
Why Choose HIMAX Electronics’ 4.8V 220mAh 2/3 AAA NiMH Batteries?
Quality Assurance
HIMAX Electronics, a professional battery supplier, prioritizes quality above all. Our 4.8V 220mAh 2/3 AAA NiMH batteries are built with premium materials and optimized internal conductors to minimize energy loss and deliver higher energy density. Every batch undergoes rigorous testing to ensure consistent performance, meeting the stringent demands of burglar alarm systems.
Customization Services
We understand that security systems often have unique requirements. HIMAX offers tailored solutions—whether adjusting battery capacity or integrating specific connectors (e.g., JR connectors)—to ensure perfect compatibility with your devices. This flexibility empowers B2B clients with versatile options.
Reliable Supply Chain
With advanced production lines and a robust supply network, HIMAX guarantees stable large-volume orders. For security companies needing long-term procurement, our dependable supply minimizes production downtime risks caused by shortages.
Technical Support & Warranty
Backed by a 2-year warranty and a dedicated technical team, HIMAX provides end-to-end support—from battery selection to troubleshooting. Our experts ensure seamless integration and peace of mind for every customer.