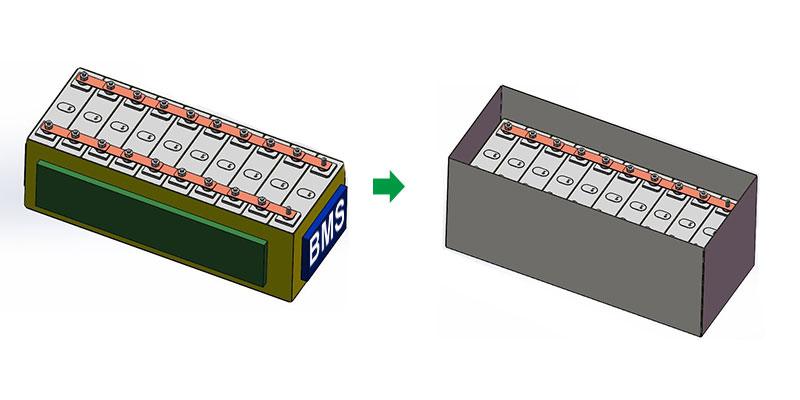
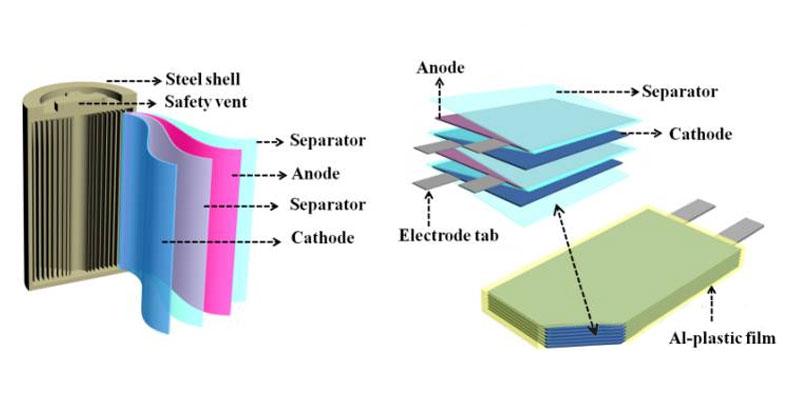
Understanding Battery ConfigurationLiFePO4 (lithium iron phosphate) batteries are a popular choice for various applications. They are known for their safety, longevity, and efficiency. The 12V battery configuration is common in renewable energy systems, electric vehicles, and portable power stations. Understanding the cell configuration in these batteries is crucial for effective use and management. This article explores the specifics of a 12V LiFePO4 battery’s cell count and highlights Himax Electronics’ role in providing high-quality battery solutions
Introduction to LiFePO4 Battery Cells
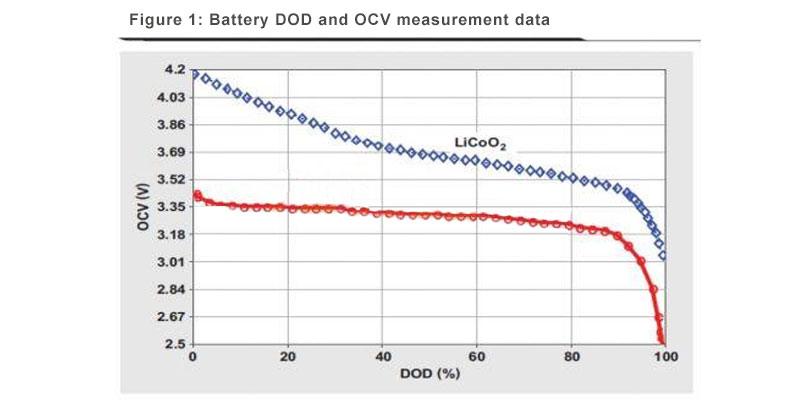
Before discussing the specific number of cells in a 12V battery, it’s important to understand what constitutes a cell in LiFePO4 batteries:Cell Basics: Each cell in a LiFePO4 battery has a nominal voltage of approximately 3.2 volts when fully charged. This is the standard voltage of a single LiFePO4 cell due to its unique chemistry and electrode configuration. Stability and Safety: LiFePO4 cells are known for their stable chemistry, which provides a higher degree of safety compared to other lithium-ion cells. They are less prone to thermal runaway and have a lower risk of fire or explosion.

Configuration of Cells in a 12V LiFePO4 Battery
To achieve a total voltage of 12 volts, multiple cells need to be combined in a specific manner:
Series Connection: A 12V LiFePO4 battery typically contains four cells connected in series. Connecting cells in series means connecting the positive terminal of one cell to the negative terminal of the next cell. This arrangement adds the voltage of each cell together while keeping the capacity (amp-hour rating) the same.Example: Four LiFePO4 cells, each with a nominal voltage of 3.2 volts, when connected in series, will cumulatively provide a voltage of 12.8 volts (4 x 3.2V = 12.8V). This is slightly higher than 12 volts, which is typical for fully charged LiFePO4 batteries.
Total Voltage and Charge Characteristics: When fully charged, each LiFePO4 cell can reach up to 3.6 volts, bringing the total voltage of a series-connected 12V battery to about 14.4 volts. During discharge, the voltage per cell can drop as low as 2.5 volts under load, which in a series configuration would decrease the total battery voltage correspondingly.
Importance of Battery Management Systems (BMS)
In battery packs like a 12V LiFePO4 battery, having an effective BMS is crucial:Voltage Regulation: The BMS ensures that all cells in the battery are charged and discharged evenly, preventing any single cell from undercharging or overcharging, which could lead to reduced battery life or failure.Safety Monitoring: It continuously monitors the voltage, temperature, and overall health of each cell, providing safeguards against potential issues like overvoltage, overheating, or short circuits.