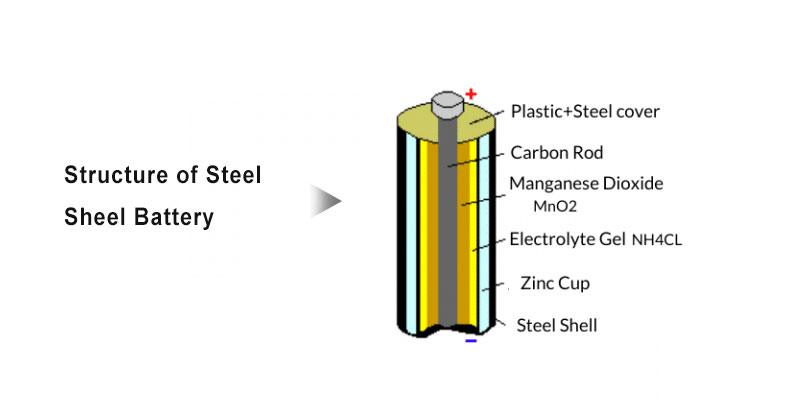
5V batteries are widely employed in various portable devices, characterized by a moderate voltage, compact size, light weight, and relatively high power output, making them an ideal energy source for many mobile devices.
Here are some common portable devices that typically utilize 5V batteries:
- Smartphones: The voltage level of 5V batteries is relatively moderate, allowing them to provide sufficient power for smartphones while maintaining a reasonable battery life. This ensures that smartphones can maintain good battery performance over an entire charging cycle.
- Tablets: Designed for lightness and portability, tablets often incorporate 5V batteries, offering a balanced power management solution to meet the performance requirements of tablets while maintaining a relatively long battery life. Similar to smartphones, tablets frequently use 5V batteries to support high-resolution screens and complex applications.
- Portable Chargers: Since most mobile devices use USB as a charging standard, and the standard voltage for USB charging is 5V, portable chargers with 5V batteries can directly support various USB charging devices, providing broader compatibility.
- Bluetooth Headphones and Earphones: Bluetooth headphones and earphones are typically low-power devices that don’t require high voltage to provide sufficient energy. The 5V battery voltage is moderate in this scenario, meeting the power needs of headphones and making them lightweight and easy to carry.
- Handheld Gaming Consoles: Designed for portability, handheld gaming consoles often use 5V batteries due to their relatively small size and lightweight, supporting extended gaming experiences.
- Smartwatches and Health Trackers: Many smartwatches and health trackers support USB charging with a standard voltage of 5V. By adopting 5V batteries, these devices can directly utilize standard USB charging cables, providing a convenient and universal charging method.
- Drones: Small and portable drones typically use 5V batteries to supply the required power for flight.
- Cameras and Camcorders: Some portable cameras and camcorders use 5V batteries, making them more convenient to carry and use.
- Handheld Electronic Devices: Including small speakers, flashlights, and mobile wireless routers, various portable electronic devices also commonly use 5V batteries.
When applying 5V batteries, it’s essential to consider the following aspects:
- Compatibility: Ensure that the selected 5V battery is compatible with the device’s voltage requirements to prevent potential damage or performance degradation.
- Quality and Reliability: Opt for high-quality and reliable brands of 5V batteries to ensure performance and safety. Low-quality batteries may pose risks such as leakage, overheating, and other safety hazards.
- Charger Selection: Use a charger that aligns with the device’s specifications in terms of charging current and voltage. Using an incorrect charger may impact battery life and safety.
- Charging Cycles: Avoid frequent deep discharge cycles, as this can accelerate the aging of 5V batteries. Regular charging and maintaining the battery at an appropriate charge level contribute to sustained performance.
- Temperature Control: Avoid using or charging 5V batteries in extreme temperatures, as extreme conditions may affect battery performance and lifespan. High temperatures can lead to overheating, while low temperatures may cause a reduction in battery capacity.
- Avoid Overdischarge and Overcharge: Prevent both full discharge and overcharging of 5V batteries. This practice helps extend the battery’s lifespan and reduce internal stress.
- Storage Conditions: If a device will not be used for an extended period, ensure the battery is fully charged before storage and store it in a cool, dry place. Avoid storing devices and batteries in environments with high temperatures or humidity.
- Maintenance Alerts: Some devices may provide maintenance alerts or settings related to battery care. It’s crucial to follow the manufacturer’s recommendations and perform maintenance promptly.
- Monitoring During Charging: Keep the device nearby during charging to take timely action in case of any abnormalities. Overcharging can lead to overheating and safety issues.
- Prevent Impact and Compression: Avoid subjecting 5V batteries to strong impacts or compression to prevent battery damage, leakage, or short circuits.
5V rechargeable batteries are a common portable power source. Through careful selection, use, and maintenance of 5V batteries, their performance can be optimized, and their lifespan extended. For more information about battery products or other advanced technological solutions, please feel free to contact us.