What is Inside a Lithium Ion Battery? An In-depth Exploration
Lithium-ion (Li-ion) batteries are integral to powering modern life, from mobile phones and laptops to electric vehicles and grid storage solutions. Understanding the components that make up these batteries is essential for appreciating their efficiency, versatility, and the cutting-edge technology behind them. This comprehensive guide details the internal workings of lithium-ion batteries and highlights the advantages of using Himax Electronics for your battery needs.
Introduction to Lithium-Ion Battery Components
A lithium-ion battery is more than just an energy storage unit; it is a complex assembly of chemistry and engineering designed to optimize energy density, longevity, and safety. Here are the key components:
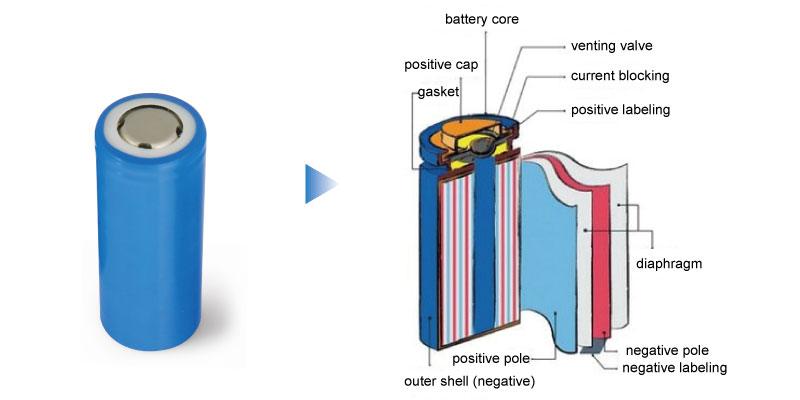
- Cathode (Positive Electrode): The cathode is a critical component that largely determines the capacity and voltage of the battery. Mading from a lithium metal oxide compound such as lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMn2O4), or newer materials like lithium iron phosphate (LiFePO4).
- Anode (Negative Electrode): The anode stores the lithium ions when the battery is charged. Commonly made from graphite, the anode allows lithium ions to embed within its structure during charging and releases them during discharge.
- Electrolyte: The electrolyte is the medium through which lithium ions move between the cathode and anode when the battery charges and discharges. It is typically composed of a lithium salt dissolved in an organic solvent.
- Separator: This porous polymer membrane plays a crucial safety role by preventing physical contact between the cathode and anode, which could lead to a short circuit while allowing ions to pass through.

How Lithium-Ion Batteries Work
The basic operation of a lithium-ion battery involves the movement of lithium ions between the anode and cathode through the electrolyte:
- During Discharge: Lithium ions flow from the anode to the cathode through the electrolyte, while electrons flow through the external circuit to the device being powered, creating an electric current.
- During Charge: The external power source forces the electrons and lithium ions back to the anode, storing energy for future use.
Benefits of Lithium-Ion Batteries
Lithium-ion batteries offer several advantages that make them the preferred choice for a wide range of applications:
- High Energy Density: Li-ion batteries provide a significant amount of energy per weight, which is particularly valuable in portable electronics and electric vehicles.
- Long Lifespan: They can typically handle hundreds to thousands of charge/discharge cycles.
- Low Self-Discharge: Unlike other battery types, Li-ion batteries lose their charge very slowly when not in use.
Challenges and Safety Considerations
Despite their advantages, Li-ion batteries come with challenges:
- Thermal Runaway Risks: If damaged or improperly managed, Li-ion batteries can overheat and lead to fires or explosions.
- Cost and Resource Intensive: The materials used in Li-ion batteries can be expensive and involve complex manufacturing processes.
Applications of Lithium-Ion Batteries
From everyday consumer electronics to critical roles in renewable energy systems and electric vehicles, lithium-ion batteries are ubiquitous in modern technology due to their efficiency and capacity.
Choosing Himax Electronics for Lithium-Ion Batteries
Himax Electronics stands out in the lithium-ion battery market for several reasons:
- Quality and Reliability: We provide top-quality lithium-ion batteries that meet rigorous performance and safety standards.
- Innovation and Technology: Our commitment to research and development ensures access to the latest advancements in battery technology.
- Expertise and Support: With extensive experience in the battery industry, Himax offers unmatched customer support and technical guidance.
Conclusion
Understanding the internal components and operation of lithium-ion batteries provides valuable insights into their functionality and widespread use. For anyone seeking reliable and high-performance lithium-ion batteries, Himax Electronics offers innovative solutions backed by expert support and quality assurance.