Introduction
The electrical system in a recreational vehicle (RV) is pivotal for ensuring comfort and convenience while traveling off-grid. A properly configured battery and inverter setup not only powers essential appliances but also enhances the overall RV experience by providing reliable access to electricity in remote locations. This article dives into how to select the correct battery and inverter combination based on specific power requirements, ensuring you never run out of power when you need it most.
In choosing the right setup, one must consider the battery’s capacity to store energy and the inverter’s ability to convert this stored energy into usable AC power. The objective is to balance these components to achieve efficient power usage without exceeding the system’s capabilities, thus avoiding potential damage or inefficiencies.
Fundamentals of RV Battery Systems
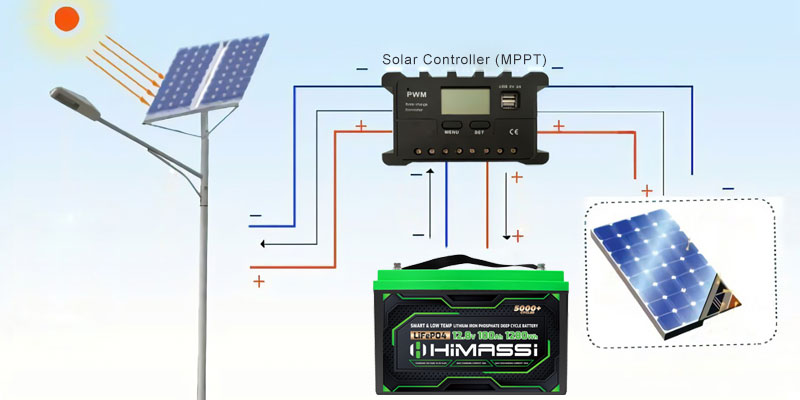
RV battery systems are designed to store a significant amount of energy that can be accessed when external power sources are not available. These systems typically involve one or more batteries coupled with an inverter that converts DC power stored in the batteries into AC power, which is the standard used by most household appliances.
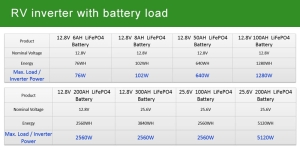
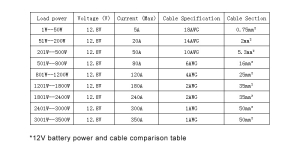
- Battery Capacity: This is typically measured in watt-hours (WH), indicating the total amount of energy the battery can store. For example, a 12.8V 200AH LiFePO4 battery holds approximately 2560WH of energy, providing substantial power for various appliances and devices.
- Inverter Output: The inverter’s role is to convert the DC energy from the batteries into AC power. The maximum load the inverter can handle is crucial for determining which appliances can be run simultaneously. For instance, the same 12.8V 200AH battery paired with a suitable inverter can efficiently manage a load of up to 2560 watts, as indicated by the specifications.
Understanding these components’ interplay helps in configuring a system that can handle typical loads like lighting, cooking appliances, and air conditioning without tripping the inverter or draining the battery too quickly.
Battery Capacity and Inverter Output Analysis
The ability of an RV battery pack to sustain a particular load is determined by both its capacity and the inverter’s power output. A well-matched battery and inverter setup allows for optimal energy usage, ensuring all appliances can operate efficiently without overloading the system. Here’s a breakdown of different battery configurations and their respective maximum load capabilities:
- 8V 6AH LiFePO4 Battery
- Nominal Voltage: 12.8V
- Energy Storage: 76WH
- Max Load/Inverter Power: 76W This configuration is suitable for very light power needs, such as charging small devices like smartphones and laptops. It’s ideal for users who require minimal power in compact setups, emphasizing portability over capacity.
- 8V 50AH LiFePO4 Battery
- Nominal Voltage: 12.8V
- Energy Storage: 640WH
- Max Load/Inverter Power: 640W This medium-range battery setup is capable of running several smaller appliances or a couple of larger ones, such as a small refrigerator or microwave oven, making it suitable for weekend trips or short adventures.
- 8V 100AH LiFePO4 Battery
- Nominal Voltage: 12.8V
- Energy Storage: 1280WH
- Max Load/Inverter Power: 1280W For more extensive energy needs, this configuration can support larger appliances and multiple smaller devices simultaneously, ideal for families or long-term travelers who need a reliable power source for various comforts and conveniences.
- 8V 200AH LiFePO4 Battery
- Nominal Voltage: 12.8V
- Energy Storage: 2560WH
- Max Load/Inverter Power: 2560W Offering substantial power, this setup is equipped to handle almost all typical RV energy needs, including air conditioning systems, heating units, and extensive kitchen appliances, suitable for full-time RV living or luxurious camping experiences.
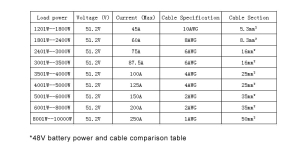
- 6V 100AH LiFePO4 Battery
- Nominal Voltage: 25.6V
- Energy Storage: 2560WH
- Max Load/Inverter Power: 2560W This higher voltage system allows for more efficient energy transfer over longer distances within the RV, reducing energy loss and improving overall system efficiency, ideal for sophisticated setups with high power demands.
- 6V 200AH LiFePO4 Battery
- Nominal Voltage: 25.6V
- Energy Storage: 5120WH
- Max Load/Inverter Power: 5120W As one of the most robust setups listed, this configuration supports the highest load capacity, capable of powering virtually everything in a large RV without compromise, including simultaneous use of multiple high-power appliances.
These configurations illustrate how varying battery capacities and inverter outputs can be tailored to specific RV needs, from minimalistic setups to full-scale living accommodations. By choosing the right combination, RV owners can ensure they have sufficient power to cover all their needs while avoiding the pitfalls of underpowered systems.
How to Choose the Right Inverter and Battery Pack
Choosing the appropriate inverter and battery pack for an RV involves understanding both the power demands of the RV’s appliances and the efficiency and capacity of the energy storage system. Here’s how to make informed choices:
- Assessing Power Requirements: Start by listing all the appliances and devices you plan to use in the RV. Calculate the total wattage these appliances require when operated simultaneously to determine the minimum inverter power needed. For example, if the combined appliances consume 2000 watts, an inverter with at least a 2200-2500 watt capacity is advisable to ensure it can handle the load without being overstressed.
- Understanding Battery Capacity Needs: The battery should have enough capacity to store sufficient energy for your usage, especially if you plan to spend extended periods off-grid. Use the total daily energy consumption to estimate the required battery storage in watt-hours (WH). For instance, if you use 2000 WH per day, a battery with at least 2560 WH (like a 12.8V 200AH battery) would be necessary to ensure a full day’s supply with some buffer.
- Choosing the Right Inverter: The inverter should not only match or exceed the total wattage requirement but also be compatible with the battery voltage. It’s crucial to select an inverter that can efficiently convert the DC power from the battery to AC power with minimal loss. Inverters with pure sine wave output are generally preferred for RVs because they provide clean, stable power that is safe for sensitive electronics and appliances.
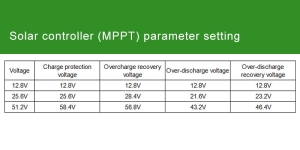
- Compatibility and Safety Features: Ensure that the battery and inverter are compatible in terms of voltage and capacity. Additionally, look for safety features in the inverter such as over-voltage protection, under-voltage shutdown, and thermal protection, which help prevent damage to both the electrical system and the connected devices.
- Installation Considerations: Proper installation is crucial for the safety and efficiency of the power system. It is recommended to have a professional install the inverter and battery system, ensuring that all connections are secure and that the system is ventilated properly to prevent overheating.
Practical Tips for Choosing Systems
- For Minimal Power Needs: A smaller setup, such as a 12.8V 50AH battery with a 640W inverter, may suffice for powering basic essentials like lighting and small appliances.
- For Moderate Use: For regular use including kitchen appliances and entertainment systems, a mid-range system like a 12.8V 100AH battery paired with a 1280W inverter is advisable.
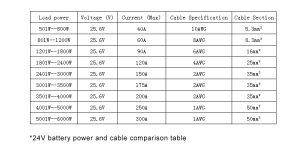
- For High Power Demands: For full-time RV living with high power requirements, including air conditioning and heating systems, opting for a more robust system such as a 25.6V 200AH battery with a 5120W inverter ensures all needs are met comfortably.
By carefully selecting the right inverter and battery pack according to these guidelines, RV owners can ensure a reliable and efficient power supply that matches their lifestyle and energy needs.
Case Studies and Real-world Applications
Understanding how different setups perform in real-life situations can provide valuable insights for RV owners considering their own system upgrades or installations. Here are several case studies demonstrating the efficacy of various battery and inverter configurations:
- Case Study 1: Weekend Warrior Setup
- Configuration: 12.8V 50AH LiFePO4 Battery with a 640W Inverter
- Usage: Primarily used for short weekend trips where the power demand includes charging devices, running a small fridge, and lighting.
- Outcome: This setup proved sufficient for the couple’s short trips, allowing them to enjoy basic comforts without the need for extensive energy reserves. The compact battery size and moderate inverter capacity kept costs and space usage minimal while adequately covering their energy needs.
- Case Study 2: Family Road Trip Adventure
- Configuration: 12.8V 200AH LiFePO4 Battery with a 2560W Inverter
- Usage: Designed for a family who spends extended periods on the road, needing to power a microwave, TV, several smartphones, and occasionally an air conditioner.
- Outcome: The robust battery capacity and high-powered inverter effortlessly handled the high and varied power demands. It provided a reliable power source throughout long trips, even supporting air conditioning use during hot days, enhancing comfort and convenience on the road.
- Case Study 3: Full-time RV Living
- Configuration: 25.6V 200AH LiFePO4 Battery with a 5120W Inverter
- Usage: Utilized by a retired couple living full-time in their RV, requiring continuous operation of heavy appliances such as a heating system, large refrigerator, and occasionally power tools.
- Outcome: This high-capacity system enabled the couple to live comfortably with all the amenities of a traditional home. The higher voltage and large capacity inverter efficiently managed the substantial and continuous power requirements, proving to be a reliable setup for full-time use.
These case studies illustrate the importance of matching the battery and inverter capacities to the specific needs and usage patterns of RV owners. Each scenario highlights how different configurations can be optimized for various lifestyles, from occasional getaways to full-time living.
Conclusion
Choosing the right battery and inverter for an RV is crucial for ensuring a reliable power supply that can handle all necessary appliances and devices without the risk of overloading or inefficient energy use. The selection process should consider the specific power requirements of the RV lifestyle, whether it’s for occasional weekend getaways or full-time living on the road. The right setup not only provides peace of mind but also enhances the overall comfort and functionality of the RV.
Through the detailed analysis of different battery capacities and inverter outputs, as well as practical case studies, it’s clear that a well-matched system can significantly improve the quality of life on the road. Each configuration serves different needs and choosing the appropriate setup depends on a careful assessment of energy demands, lifestyle, and budget.
About Himax Electronics
Himax Electronics stands at the forefront of renewable energy solutions, specializing in high-performance batteries and inverters that cater to a wide range of applications, including RV systems. With a focus on innovation, reliability, and customer satisfaction, Himax is dedicated to providing top-tier products that enhance energy independence and sustainability.
- Innovative Solutions: Himax Electronics continuously develops cutting-edge technology designed to improve energy efficiency and storage capabilities. Our products are built to meet the highest standards of quality and performance, ensuring they withstand the rigors of both occasional and constant use.
- Sustainable Practices: Committed to environmental stewardship, Himax promotes the use of renewable energy sources to reduce carbon footprints and foster sustainable living practices. Our battery systems are designed to offer long life spans and high efficiency, contributing to a greener planet.
- Customer-Centric Approach: Understanding that each customer’s needs are unique, Himax offers personalized consultation services to help RV owners find the perfect energy solution. Our team provides expert advice and support throughout the selection, installation, and maintenance processes, ensuring that each customer achieves their desired level of energy independence.
In conclusion, whether you are an occasional camper or a full-time RVer, Himax Electronics provides the tools and technology needed to power your adventures reliably and sustainably. Embrace the freedom of the open road with confidence, knowing that your energy needs are met with the highest standards of efficiency and environmental responsibility.