Charging at High and Low Temperatures
Learn how to extend battery life by moderating ambient temperatures.
Batteries operate over a wide temperature range, but this does not give permission to also charge them at these conditions. The charging process is more delicate than discharging and special care must be taken. Extreme cold and high heat reduce charge acceptance, so the battery must be brought to a moderate temperature before charging.
Older battery technologies, such as lead acid and NiCd, have higher charging tolerances than newer systems. This allows them to charge below freezing but at a reduced charge C-rate. When it comes to cold-charging NiCd is hardier than NiMH.
Table 1 summarizes the permissible charge and discharge temperatures of common rechargeable batteries. The table excludes specialty batteries that are designed to charge outside these parameters.
|
Battery type |
Charge temperature |
Discharge temperature |
Charge advisory |
|
Lead acid |
–20°C to 50°C (–4°F to 122°F) |
–20°C to 50°C (–4°F to 122°F) |
Charge at 0.3C or lessbelow freezing. Lower V-threshold by 3mV/°C when hot. |
|
NiCd, NiMH |
0°C to 45°C (32°F to 113°F) |
–20°C to 65°C (–4°F to 149°F) |
Charge at 0.1C between –18°C and 0°C.
Charge at 0.3C between 0°C and 5°C. |
|
Li-ion |
0°C to 45°C (32°F to 113°F) |
–20°C to 60°C (–4°F to 140°F) |
No charge permitted below freezing. Good charge/discharge performance at higher temperature but shorter life. |
Table 1: Permissible temperature limits for various batteries. Batteries can be discharged over a large temperature range, but the charge temperature is limited. For best results, charge between 10°C and 30°C (50°F and 86°F). Lower the charge current when cold.
Low-temperature Charge
Fast charging of most batteries is limited to 5°C to 45°C (41°F to 113°F); for best results consider narrowing the temperature bandwidth to between 10°C and 30°C (50°F and 86°F) as the ability to recombine oxygen and hydrogen diminishes when charging nickel-based batteries below 5°C (41°F). If charged too quickly, pressure builds up in the cell that can lead to venting. Reduce the charge current of all nickel-based batteries to 0.1C when charging below freezing.
Nickel-based chargers with NDV full-charge detection offer some protection when fast charging at low temperatures; the poor charge acceptance when cold mimics a fully charged battery. This is in part caused by a high pressure buildup due to the reduced ability to recombine gases at low temperature. Pressure rise and a voltage drop at full charge appear synonymous.
To enable fast charging at all temperatures, some industrial batteries add a thermal blanket that heats the battery to an acceptable temperature; other chargers adjust the charge rate to prevailing temperatures. Consumer chargers do not have these provisions and the end user is advised to only charge at room temperature.
Lead acid is reasonably forgiving when it comes to temperature extremes, as the starter batteries in our cars reveal. Part of this tolerance is credited to their sluggish behavior. The recommended charge rate at low temperature is 0.3C, which is almost identical to normal conditions. At a comfortable temperature of 20°C (68°F), gassing starts at charge voltage of 2.415V/cell. When going to –20°C (0°F), the gassing threshold rises to 2.97V/cell.
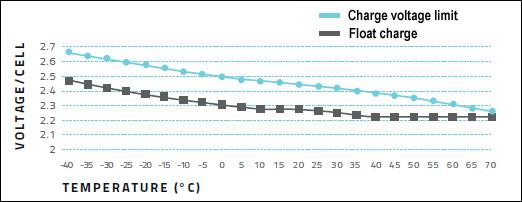
A lead acid battery charges at a constant current to a set voltage that is typically 2.40V/cell at ambient temperature. This voltage is governed by temperature and is set higher when cold and lower when warm. Figure 2 illustrates the recommended settings for most lead acid batteries. In parallel, the figure also shows the recommended float charge voltage to which the charger reverts when the battery is fully charged. When charging lead acid at fluctuating temperatures, the charger should feature voltage adjustment to minimize stress on the battery. (See also BU-403: Charging Lead Acid.)
Figure 2: Cell voltages on charge and float at various temperatures.
Charging at cold and hot temperatures requires adjustment of voltage limit.
Freezing a lead acid battery leads to permanent damage. Always keep the batteries fully charged because in the discharged state the electrolyte becomes more water-like and freezes earlier than when fully charged. According to BCI, a specific gravity of 1.15 has a freezing temperature of –15°C (5°F). This compares to –55°C (–67°F) for a specific gravity of 1.265 with a fully charged starter battery. Flooded lead acid batteries tend to crack the case and cause leakage if frozen; sealed lead acid packs lose potency and only deliver a few cycles before they fade and need replacement.
Li ion can be fast charged from 5°C to 45°C (41 to 113°F). Below 5°C, the charge current should be reduced, and no charging is permitted at freezing temperatures because of the reduced diffusion rates on the anode. During charge, the internal cell resistance causes a slight temperature rise that compensates for some of the cold. The internal resistance of all batteries rises when cold, prolonging charge times noticeably.
Many battery users are unaware that consumer-grade lithium-ion batteries cannot be charged below 0°C (32°F). Although the pack appears to be charging normally, plating of metallic lithium can occur on the anode during a sub-freezing charge. This is permanent and cannot be removed with cycling. Batteries with lithium plating are more vulnerable to failure if exposed to vibration or other stressful conditions. Advanced chargers (Cadex) prevent charging Li-ion below freezing.
Advancements are being made to charge Li-ion below freezing temperatures. Charging is indeed possible with most lithium-ion cells but only at very low currents. According to research papers, the allowable charge rate at –30°C (–22°F) is 0.02C. At this low current, the charge time would stretch to over 50 hours, a time that is deemed impractical. There are, however, specialty Li-ions that can charge down to –10°C (14°F) at a reduced rate.
High-temperature Charge
Heat is the worst enemy of batteries, including lead acid. Adding temperature compensation on a lead acid charger to adjust for temperature variations is said to prolong battery life by up to 15 percent. The recommended compensation is a 3mV drop per cell for every degree Celsius rise in temperature. If the float voltage is set to 2.30V/cell at 25°C (77°F), the voltage should read 2.27V/cell at 35°C (95°F). Going colder, the voltage should be 2.33V/cell at 15°C (59°F). These 10°C adjustments represent 30mV change.
Table 3 indicates the optimal peak voltage at various temperatures when charging lead acid batteries. The table also includes the recommended float voltage while in standby mode.
| BATTERY STATUS | -40°C (-40°F) | -20°C (-4°F) | 0°C (32°F) | 25°C (77°F) | 40°C (104°F) |
|---|---|---|---|---|---|
| Voltage limit on recharge |
2.85V/cell | 2.70V/cell | 2.55V/cell | 2.45V/cell | 2.35V/cell |
| Float voltage at full charge |
2.55V/cell or lower |
2.45V/cell or lower |
2.35V/cell or lower |
2.30V/cell or lower |
2.25V/cell or lower |
Table 3: Recommended voltage limits when charging and maintaining stationary lead acid batteries on float charge. Voltage compensation prolongs battery life when operating at temperature extremes.
Charging nickel-based batteries at high temperatures lowers oxygen generation, which reduces charge acceptance. Heat fools the charger into thinking that the battery is fully charged when it’s not.
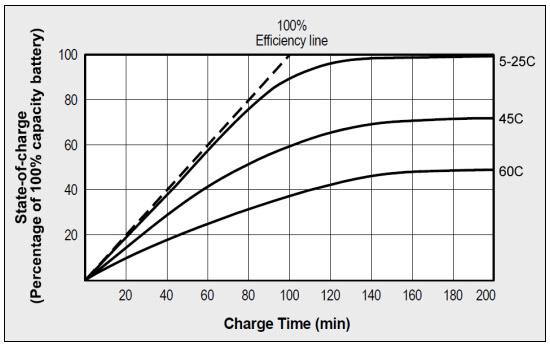
Charging nickel-based batteries when warm lowers oxygen generation that reduces charge acceptance. Heat fools the charger into thinking that the battery is fully charged when it’s not. Figure 4 shows a strong decrease in charge efficiency from the “100 percent efficiency line” when dwelling above 30°C (86°F). At 45°C (113°F), the battery can only accept 70 percent of its full capacity; at 60°C (140°F) the charge acceptance is reduced to 45 percent. NDV for full-charge detection becomes unreliable at higher temperatures, and temperature sensing is essential for backup.
Figure 4: NiCd charge acceptance as a function of temperature. High temperature reduces charge acceptance and departs from the dotted “100% efficiency line.” At 55°C, commercial NiMH has a charge efficiency of 35–40%; newer industrial NiMH attains 75–80%.
Lithium-ion performs well at elevated temperatures but prolonged exposure to heat reduces longevity. Charging and discharging at elevated temperatures is subject to gas generation that might cause a cylindrical cell to vent and a pouch cell to swell. Many chargers prohibit charging above 50°C (122°F).
Some lithium-based packs are momentarily heated to high temperatures. This applies to batteries in surgical tools that are sterilized at 137°C (280°F) for up to 20 minutes as part of autoclaving. Oil and gas drilling as part of fracking also exposes the battery to high temperatures.
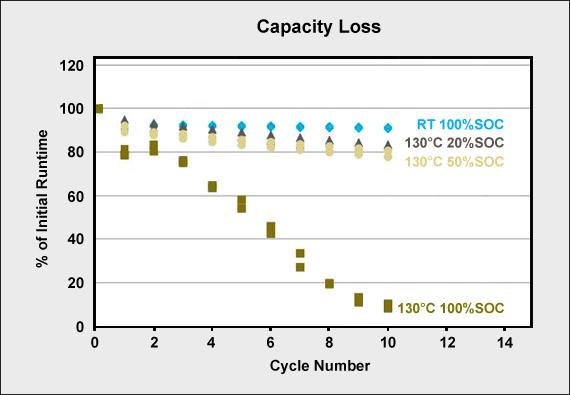
Capacity loss at elevated temperature is in direct relationship with state-of-charge (SoC). Figure 5 illustrates the effect of Li-cobalt (LiCoO2) that is first cycled at room temperature (RT) and then heated to 130°C (266°F) for 90 minutes and cycled at 20, 50 and 100 percent SoC. There is no noticeable capacity loss at room temperature. At 130°C with a 20 percent SoC, a slight capacity loss is visible over 10 cycles. This loss is higher with a 50 percent SoC and shows a devastating effect when cycled at full charge.
Figure 5: Capacity loss at room temperature (RT) and 130°C for 90 minutes
Sterilization of batteries for surgical power tools should be done at low SoC.
Test: LiCoO2/Graphite cells were exposed to 130°C for 90 min.at different SoC between each cycle.