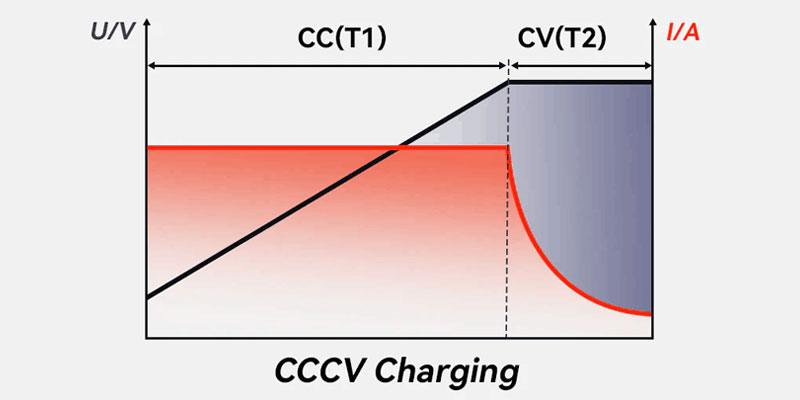
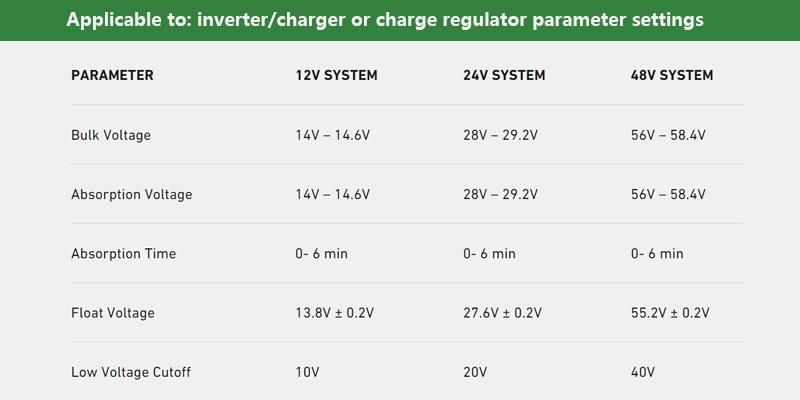
Introduction
Battery performance in cold environments is a critical issue that affects not only the efficiency but also the operational viability of many modern technologies. In regions where temperatures regularly fall below freezing, conventional batteries can struggle, significantly impacting the functionality of everything from electric vehicles to remote sensors and renewable energy storage systems. The key challenge lies in the battery’s chemical composition and the physics of its operation: cold temperatures slow the kinetic energy of the molecules within the battery, reducing the rate at which chemical reactions occur, which is essential for charging and discharging. Moreover, the Battery Management System (BMS), designed to protect the battery’s integrity, often compounds these issues by preventing charging to avoid damage when it detects temperatures that are too low.
This article aims to demystify the problems associated with charging low-temperature protection batteries and to explore practical solutions that can mitigate these effects. By understanding the underlying causes and implementing strategic interventions, users can enhance battery performance even in harsh winter conditions, ensuring reliability and extending the lifespan of their battery-powered devices.

Common Reasons Why Low-Temperature Protection Batteries Fail to Charge
- Impeded Internal Chemical Reactions: At lower temperatures, the electrolyte within the battery thickens, slowing the mobility of lithium ions that travel between the cathode and anode during charging and discharging processes. This decreased ionic mobility drastically reduces the battery’s ability to accept and hold a charge. Additionally, the lower temperatures can cause an increase in the internal resistance of the battery, further reducing its efficiency and increasing the time required to charge fully.
- Limitations of Battery Management Systems (BMS): The BMS is essentially the brain of the battery, designed to ensure safe operation by monitoring and controlling battery parameters such as voltage, current, and temperature. In cold conditions, many BMS are programmed to prevent charging when the battery temperature falls below a specific limit, typically around 0°C. This protective measure is intended to prevent damage from charging a battery when the electrolyte is too sluggish to facilitate proper ion transfer, which could lead to incomplete charging cycles and, over time, battery degradation.
- External Factors: The performance of the charging equipment itself can also be a limiting factor in cold environments. Chargers and cables not designed for cold weather may become less efficient or fail to operate altogether. For instance, the materials used in some chargers and cables can become brittle and lose conductivity at low temperatures, further complicating the charging process. Additionally, the ambient cold can exacerbate the issue by cooling the battery even further during charging, especially if the charging setup lacks proper insulation.
Understanding these common causes provides a foundation for exploring effective solutions to enhance battery charging under cold conditions, ensuring that devices remain functional and reliable, no matter the external temperature.
Technical Solutions and Strategies
To counteract the challenges posed by low temperatures, several technical solutions and strategies can be implemented to improve battery charging efficiency and reliability:
- Heating Technologies: One of the most direct methods to address low-temperature charging issues is the integration of heating systems within the battery setup. These can include external heating pads or internal heating elements that activate before and during the charging process. By slightly warming the batteries, these heaters bring the internal battery temperature to a minimal acceptable level for efficient charging. This not only improves the charging rate but also helps maintain the battery’s capacity and health over time.
- Adjusting BMS Settings: Modifying the Battery Management System (BMS) parameters to better suit cold environments can make a significant difference. This might involve recalibrating the BMS to allow charging at lower temperatures or to control the rate of charging based on the temperature of the battery. Advanced BMS can also dynamically adjust charging characteristics in response to real-time temperature readings, optimizing charging rates and improving battery longevity.
- Using Appropriate Charging Equipment: Selecting chargers and cables that are specifically designed to perform in cold conditions is crucial. These devices are built with materials that retain flexibility and conductivity even at low temperatures. Additionally, they may include enhanced insulation to protect against the cold, ensuring that the maximum amount of energy is efficiently transferred to the battery without thermal losses.
Implementing these solutions requires a careful assessment of the existing battery infrastructure and may involve initial setup costs. However, the long-term benefits of maintaining operational efficiency and battery health in cold climates far outweigh these initial investments. These strategies not only enhance the functionality of batteries in cold environments but also extend their usable life, making them more cost-effective over time.
Case Studies
To illustrate the effectiveness of the solutions and strategies discussed, let’s examine a few real-world applications where these methods have been successfully implemented to solve low-temperature charging problems:
Case Study 1: Remote Weather Station in Alaska
- Problem: A remote weather station in Alaska faced significant challenges with battery performance during the winter months, with temperatures often dropping below -30°C. The station relied on these batteries for critical weather monitoring and data transmission.
- Solution: The station implemented external battery heaters connected to a solar-powered system, ensuring the batteries remained within an operational temperature range. Additionally, the BMS settings were adjusted to allow for slower charging rates during extremely cold periods.
- Outcome: The modifications led to a noticeable improvement in battery reliability and a reduction in power failures during critical weather events, enhancing the station’s operational continuity throughout the winter.
Case Study 2: Electric Vehicle Fleet in Norway
- Problem: An electric vehicle (EV) fleet operator in Norway reported reduced range and slower charging speeds during the winter season, affecting the fleet’s efficiency and reliability.
- Solution: The EV company integrated internal battery heating systems that pre-warmed the batteries before charging commenced. They also upgraded their charging stations with cables and connectors designed for low temperatures.
- Outcome: These changes resulted in faster charging times and more consistent battery performance, significantly reducing downtime and increasing the daily operational range of the vehicles.
Case Study 3: Solar-Powered Sensor Network in the Himalayas
- Problem: A network of solar-powered sensors placed in the Himalayas to monitor glacial movements struggled with battery charging issues due to the frigid temperatures, which often caused system failures.
- Solution: Each sensor unit was equipped with a small, insulated battery compartment featuring a low-energy internal heater. The BMS was specially programmed to manage power use efficiently, prioritizing battery heating and charging based on solar input.
- Outcome: The enhanced system provided a stable power supply throughout the year, increasing data reliability and sensor uptime, crucial for long-term climate studies.
These case studies demonstrate the tangible benefits of implementing targeted solutions to address low-temperature battery charging challenges. By adopting similar strategies, organizations can ensure their battery-dependent technologies remain functional and efficient, regardless of the environmental conditions.
User Guide and Best Practices
For individuals and organizations managing battery systems in cold environments, following these best practices can significantly improve battery performance and longevity:
- Preconditioning Batteries:
- Purpose: Preconditioning involves bringing the battery up to an optimal temperature before beginning the charging process. This practice can be especially effective in maintaining battery health and efficiency.
- Method: Use built-in heating systems or external warming devices to gently heat the battery. If the system allows, automate this process so that it occurs just before the expected charging time.
- Regular Maintenance and Inspections:
- Routine Checks: Regularly inspect battery installations for signs of wear, insulation failures, or damage to heating elements and connections. Cold weather can exacerbate existing issues or introduce new vulnerabilities.
- Scheduled Maintenance: Establish a maintenance schedule that considers the environmental stressors typical of your operation’s location. This may include more frequent checks during the winter months.
- Optimizing Charging Times and Conditions:
- Charging Windows: Where possible, plan to charge batteries during the warmest part of the day or when they have been active and naturally warmed through use.
- Charging Rate Adjustments: Lower the charge rate to accommodate slower chemical reactions at lower temperatures, which can help preserve battery capacity and reduce strain.
- Using Suitable Insulation:
- Insulation Materials: Protect battery systems with insulation that can withstand the specific conditions of your environment. Materials should be durable, moisture-resistant, and capable of minimizing thermal loss.
- Design Considerations: Ensure that battery enclosures and installations are designed to minimize exposure to cold winds and moisture, which can freeze components and reduce efficiency.
- Battery Storage:
- Short-Term Storage: If batteries are not in use, store them in a controlled environment where temperature fluctuations are minimized. Avoid allowing the battery to sit at low charge levels for extended periods in cold conditions.
- Long-Term Storage: For batteries stored over longer periods, maintain a charge level recommended by the manufacturer and consider periodic recharging to keep the battery healthy.
By implementing these practices, users can effectively manage the challenges posed by cold environments, ensuring that their battery systems remain operational and efficient throughout their service life. These strategies not only safeguard the equipment but also optimize energy usage and operational costs.

About Himax Electronics
Himax Electronics is a leading innovator in the battery technology sector, specializing in the development and manufacture of high-performance LiFePO4 batteries(LIFEPO4 BATTERY) suited for a wide array of applications, including those requiring robust low-temperature operation. Our commitment to excellence and innovation is evident in every product we design and every solution we provide to our customers.
Product Range and Custom Solutions:
- We offer a comprehensive range of battery products, from standard models to custom-designed units that meet specific operational requirements, including those needed for extreme environmental conditions. Our low-temperature batteries are engineered with advanced materials and technologies that provide reliable performance even under the harshest conditions.
Quality and Reliability:
- At Himax Electronics, quality assurance is paramount. Our batteries undergo rigorous testing processes to meet high standards of durability and performance. We adhere to international safety and quality standards, ensuring our products deliver longevity and reliability for critical applications across all industries.
Customer-Centric Support and Innovation:
- We pride ourselves on our customer-centric approach, providing tailored solutions that fit the unique needs of each client. Whether you’re facing challenges in cold climates or need a battery that can withstand unusual environmental stressors, our team is ready to assist with expert advice, technical support, and post-sale service.
- Our commitment to innovation extends beyond our products. We are continually researching and developing new technologies to enhance battery efficiency, extend lifespans, and reduce environmental impact, ensuring our customers receive the most advanced battery solutions available.
Sustainability and Environmental Responsibility:
- Environmental stewardship is integral to our business philosophy. We strive to minimize our ecological footprint by implementing sustainable practices in our manufacturing processes and by designing products that are both energy-efficient and recyclable.
Himax Electronics is more than just a battery supplier; we are a partner in your energy journey. We invite you to explore our diverse product offerings and discover how our cutting-edge battery solutions can empower your applications. For more detailed information about our products and services or to discuss a custom battery solution, please visit our website or contact our dedicated customer service team. We are here to power your success with reliable, innovative, and responsible energy solutions.