Introduction to Portable Gas Detectors
Portable gas detectors are essential safety devices widely used across various industries including oil & gas, mining, chemical plants, laboratories, and manufacturing facilities. These compact, handheld instruments continuously monitor the surrounding air for the presence of hazardous gases such as carbon monoxide (CO), hydrogen sulfide (H2S), oxygen (O2), methane (CH4), and volatile organic compounds (VOCs).
By providing real-time gas concentration data, portable gas detectors help protect workers from toxic exposure, prevent explosions, and ensure compliance with safety regulations. As these devices are used in potentially hazardous and remote environments, they rely heavily on high-performance, reliable, and safe power sources.
As a battery manufacturer with over 12 years of experience, HIMAX ELECTRONICS understands the critical role batteries play in the performance and safety of portable gas detectors. In this article, we will explore the types of batteries commonly used in these devices, and explain why our Li-Polymer (LiPo) and 18650 Li-ion batteries are optimal choices.
What Types of Batteries Are Used in Portable Gas Detectors?
The battery is a crucial component in portable gas detectors because these devices are often used in field operations where continuous and reliable power is essential. The following table summarizes the most common battery types used in gas detection equipment:
| Battery Type | Features | Limitations |
| Alkaline | Low cost, widely available | Non-rechargeable, short lifespan, environmental waste |
| NiMH (Nickel-Metal Hydride) | Rechargeable, moderate cost | Lower energy density, self-discharge issues |
| Li-ion (Lithium-Ion, including 18650 cells) | High energy density, long cycle life, lightweight | Requires safety circuits, higher initial cost |
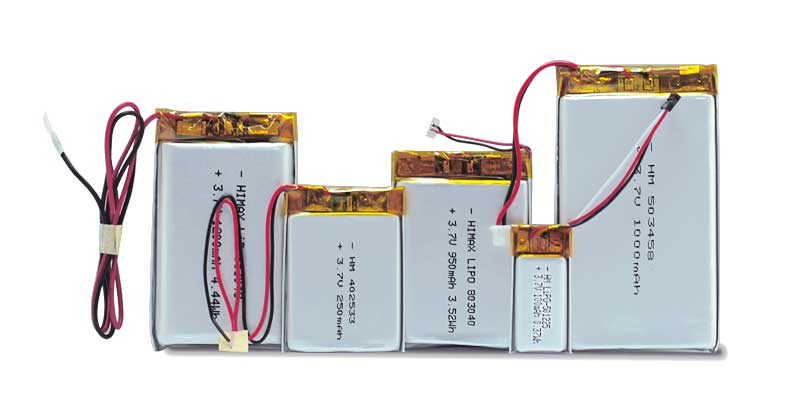
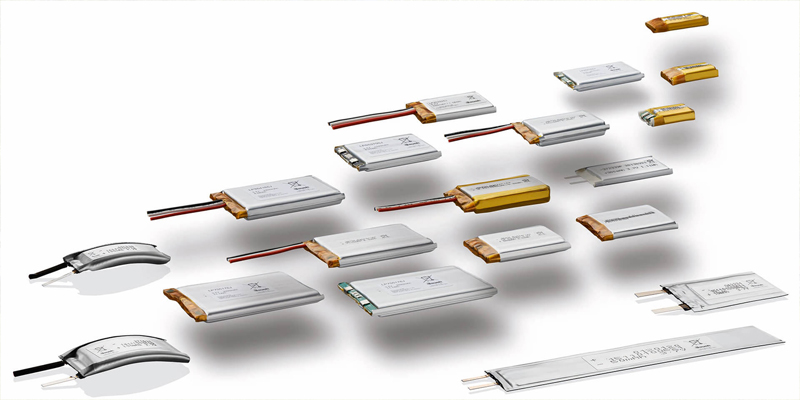
| Li-Polymer (LiPo) | Flexible shapes and sizes, high safety, high energy density | Slightly more expensive than cylindrical cells |
 In recent years, rechargeable lithium-based batteries (both Li-ion 18650 and Li-Polymer) have become the preferred choice for most modern portable gas detectors due to their superior performance and safety characteristics.
In recent years, rechargeable lithium-based batteries (both Li-ion 18650 and Li-Polymer) have become the preferred choice for most modern portable gas detectors due to their superior performance and safety characteristics.
Why Li-Polymer and 18650 Li-ion Batteries Are Ideal for Portable Gas Detectors
1. High Energy Density and Long Runtime
Portable gas detectors often operate for extended periods, sometimes 8-12 hours per shift or continuously for days in industrial environments. Batteries with high energy density ensure the device remains operational without frequent recharging or battery replacement.
| Battery Type | Energy Density (Wh/kg) |
| Alkaline | ~100 |
| NiMH | ~60-120 |
| Li-ion 18650 | ~150-260 |
| Li-Polymer | ~200-300 |
As shown, Li-Polymer and 18650 Li-ion batteries offer significantly higher energy density compared to traditional chemistries. This translates into longer operating hours and reduced downtime.
2. Rechargeable and Eco-Friendly
Rechargeable batteries not only reduce long-term costs but also minimize environmental waste compared to disposable batteries. Both Li-Polymer and 18650 Li-ion batteries can handle hundreds to thousands of charge-discharge cycles, making them highly sustainable.
| Battery Type | Typical Cycle Life |
| Alkaline | Single-use |
| NiMH | 300-500 cycles |
| Li-ion 18650 | 500-1000 cycles |
| Li-Polymer | 500-1200 cycles |
3. Compact Size and Flexible Design
Portable gas detectors need to be lightweight, compact, and ergonomic. Li-Polymer batteries offer excellent design flexibility since they can be customized into various shapes and sizes to fit specific device dimensions.
| Battery Type | Shape Flexibility |
| Alkaline | Fixed shapes |
| NiMH | Cylindrical only |
| Li-ion 18650 | Cylindrical only |
| Li-Polymer | Customizable shapes |
This flexibility allows OEM manufacturers to design slim, handheld devices without compromising battery capacity.
4. High Safety Standards
Safety is paramount for devices operating in potentially explosive environments. Modern Li-Polymer and 18650 Li-ion batteries are equipped with multiple safety mechanisms including:
- Overcharge protection
- Over-discharge protection
- Short-circuit protection
- Thermal protection
At HIMAX ELECTRONICS, our batteries undergo rigorous aging equipment tests, automatic welding, and comprehensive quality control to ensure maximum safety and reliability for industrial applications.
5. Reliable Performance in Extreme Conditions
Industrial environments often expose equipment to wide temperature ranges. Our lithium batteries are engineered to perform consistently under extreme conditions.
| Battery Type | Operating Temperature Range |
| Li-ion 18650 | -20°C to +60°C |
| Li-Polymer | -20°C to +60°C |
This temperature tolerance ensures reliable performance whether used in hot factories or cold outdoor operations.
HIMAX ELECTRONICS: Your Reliable Battery Manufacturer for Portable Gas Detectors
At HIMAX ELECTRONICS, we specialize in manufacturing high-quality rechargeable batteries with over 13 years of industry experience. Our factory is equipped with advanced production lines, automatic welding machines, and state-of-the-art aging and testing equipment to ensure consistent quality and safety.
Our Key Battery Products for Portable Gas Detectors
| Battery Model | Type | Voltage | Capacity | Features |
| HIMAX 18650 3.7V 2600mAh | Li-ion 18650 | 3.7V | 2600mAh | High energy density, long cycle life, stable output |
| HIMAX 18650 3.7V 3400mAh | Li-ion 18650 | 3.7V | 3400mAh | Higher capacity for longer runtime |
| HIMAX LP503759 | Li-Polymer | 3.7V | 1200mAh | Ultra-slim design for compact devices |
| HIMAX LP803860 | Li-Polymer | 3.7V | 2000mAh | High capacity in a thin profile |
Why Choose HIMAX ELECTRONICS as Your Battery Partner?
- Factory-direct supplywith competitive pricing
- Fully customized battery solutions to fit your device design
- Strict quality control with professional testing equipment
- Compliance with international safety certifications (UL, CE, UN38.3, RoHS)
- Fast delivery and flexible production capacity
Conclusion
Choosing the right battery for your portable gas detector is critical to ensure safety, reliability, and cost-efficiency. Both Li-Polymer and 18650 Li-ion batteries offer superior advantages in terms of energy density, rechargeability, safety, and design flexibility.
As a battery manufacturer with extensive experience, HIMAX ELECTRONICS is your trusted partner for high-quality, factory-direct battery solutions tailored for portable gas detection equipment. Contact us today to discuss your specific requirements and receive a customized battery proposal.