Learn How to Arrange Batteries to Increase Voltage or Gain Higher Capacity
Batteries achieve the desired operating voltage by connecting several cells in series; each cell adds its voltage potential to derive at the total terminal voltage. Some packs may consist of a combination of series and parallel connections. Laptop batteries commonly have four 3.6V Li-ion cells in series to achieve a nominal voltage 14.4V and two in parallel to boost the capacity from 2,400mAh to 4,800mAh. Such a configuration is called 4s2p, meaning four cells in series and two in parallel.
It is important to use the same battery type with equal voltage and capacity (Ah) and never to mix different makes and sizes. A weaker cell would cause an imbalance, as a battery is only as strong as the weakest link in the chain.
Single Cell Applications
The single-cell configuration is the simplest battery pack; the cell does not need matching and the protection circuit on a small Li-ion cell can be kept simple. Typical examples are mobile phones and tablets with one 3.60V Li-ion cell. Other uses of a single cell are wall clocks, which typically use a 1.5V alkaline cell, wristwatches and memory backup, most of which are very low power applications.
Series Connection
Portable equipment needing higher voltages use battery packs with two or more cells connected in series.

Figure 2: Series connection of four cells (4s).
Adding cells in a string increases the voltage; the capacity remains the same.
High voltage batteries keep the conductor size small. Cordless power tools run on 12V and 18V batteries; high-end models use 24V and 36V. Most e-bikes come with 36V Li-ion, some are 48V. The car industry wanted to increase the starter battery from 12V (14V) to 36V, better known as 42V, by placing 18 lead acid cells in series.
Some mild hybrid cars run on 48V Li-ion and use DC-DC conversion to 12V for the electrical system.
Parallel Connection
If higher currents are needed and larger cells are not available or do not fit the design constraint, one or more cells can be connected in parallel. Most battery chemistries allow parallel configurations with little side effect.
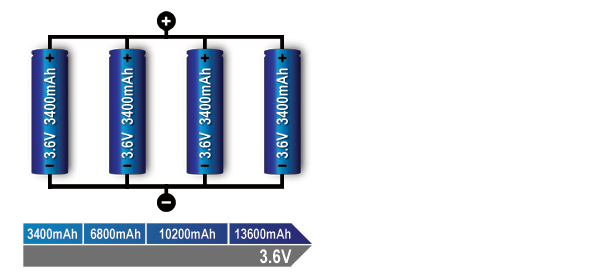
Figure 4: Parallel connection of four cells (4p).
With parallel cells, capacity in Ah and runtime increases while the voltage stays the same.
Series/parallel Connection
The series/parallel configuration shown in Figure 6 enables design flexibility and achieves the desired voltage and current ratings with a standard cell size. The total power is the product of voltage-times-current; four 3.6V (nominal) cells multiplied by 3,400mAh produce 12.24Wh. Four 18650 Energy Cells of 3,400mAh each can be connected in series and parallel as shown to get 7.2V nominal and 12.24Wh. The slim cell allows flexible pack design but a protection circuit is needed.
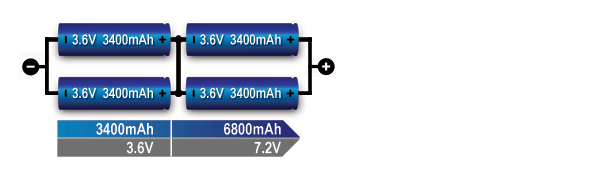
Figure 6: Series/ parallel connection of four cells (2s2p).
This configuration provides maximum design flexibility. Paralleling the cells helps in voltage management.
Safety devices in Series and Parallel Connection
Positive Temperature Coefficient Switches (PTC) and Charge Interrupt Devices (CID) protect the battery from overcurrent and excessive pressure. While recommended for safety in a smaller 2- or 3-cell pack with serial and parallel configuration, these protection devices are often being omitted in larger multi-cell batteries, such as those for power tool.
Simple Guidelines for Using Household Primary Batteries
- Keep the battery contacts clean. A four-cell configuration has eight contacts and each contact adds resistance (cell to holder and holder to next cell).
- Never mix batteries; replace all cells when weak. The overall performance is only as good as the weakest link in the chain.
- Observe polarity. A reversed cell subtracts rather than adds to the cell voltage.
- Remove batteries from the equipment when no longer in use to prevent leakage and corrosion. This is especially important with zinc-carbon primary cells.
- Do not store loose cells in a metal box. Place individual cells in small plastic bags to prevent an electrical short. Do not carry loose cells in your pockets.
- Keep batteries away from small children. In addition to being a choking hazard, the current-flow of the battery can ulcerate the stomach wall if swallowed. The battery can also rupture and cause poisoning.
- Do not recharge non-rechargeable batteries; hydrogen buildup can lead to an explosion. ·Perform experimental charging only under supervision.
Simple Guidelines for Using Secondary Batteries
- Observe polarity when charging a secondary cell. Reversed polarity can cause an electrical short, leading to a hazardous condition.
- Remove fully charged batteries from the charger. A consumer charger may not apply the correct trickle charge when fully charged and the cell can overheat.
- Charge only at room temperature.


