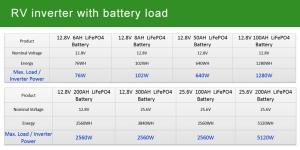
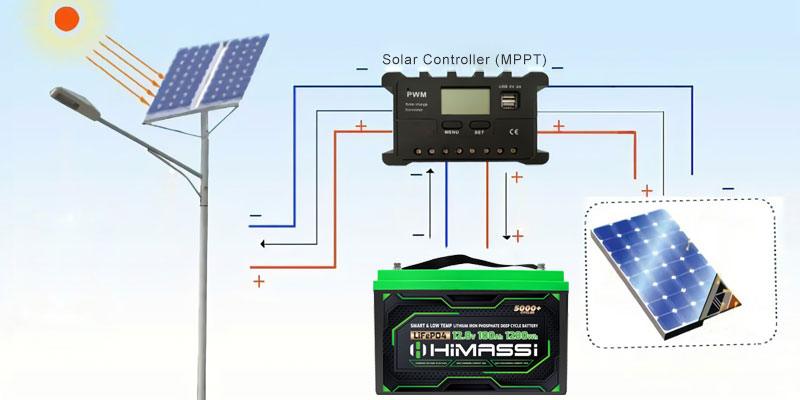
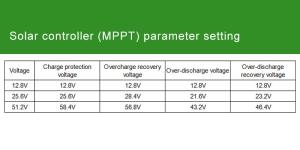
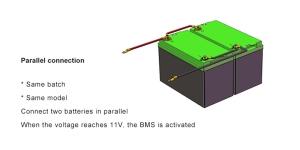
Introduction
In the rapidly evolving landscape of battery technologies, Lithium Iron Phosphate (LiFePO4) batteries have carved a niche in the US and European markets for their superior performance, safety, and environmental benefits. As industries continue to shift towards more sustainable and reliable energy solutions, understanding the strategic benefits of wholesaling LiFePO4 batteries is becoming crucial for businesses looking to enhance their operational efficiency and sustainability. This article will explore the myriad advantages of purchasing LiFePO4 batteries in bulk, offering insights into how businesses can leverage these benefits to gain a competitive edge in an increasingly eco-conscious market.

Basic Characteristics of LiFePO4 Batteries
LiFePO4 batteries are celebrated for their robust safety features, exceptional longevity, and minimal environmental impact, setting them apart from traditional lithium-ion batteries. These batteries employ lithium iron phosphate as the cathode material, which endows them with a stable chemical composition that enhances safety and performance. This section will delve into the distinct characteristics of LiFePO4 batteries that make them a preferred choice among various high-demand applications.
Safety Features
LiFePO4 batteries are inherently safe due to their stable phosphate chemistry, which significantly reduces the risk of thermal runaway—a common issue in other lithium battery types. This safety feature is crucial in applications where battery failure can lead to catastrophic consequences, such as in electric vehicles or large-scale energy storage systems. Their robust structure also ensures that they are less prone to catching fire or exploding, even under harsh conditions, making them a reliable choice for critical applications.
Stability
The structural stability of LiFePO4 batteries allows them to maintain consistent performance over a wide range of temperatures, from freezing conditions to extreme heat. Unlike other batteries that degrade rapidly under adverse conditions, LiFePO4 batteries exhibit minimal capacity loss, ensuring long-term reliability and efficiency. This stability not only enhances their lifespan but also reduces the frequency of replacements, providing significant cost savings over time.
Environmental Impact
One of the most compelling attributes of LiFePO4 batteries is their environmental friendliness. Made from non-toxic materials, these batteries pose minimal risk to the environment compared to their lead-acid and other lithium-based counterparts. Their high recyclability further underscores their green credentials, aligning with global efforts to promote sustainable and eco-friendly technologies.
Benefits of Purchasing LiFePO4 Batteries Wholesale
The decision to purchase LiFePO4 batteries wholesale can be driven by numerous factors, including cost-effectiveness, performance advantages, and environmental benefits. This section expands on the economic and operational incentives that make bulk purchasing an attractive option for businesses.
Cost-Effectiveness
Investing in LiFePO4 batteries wholesale is economically advantageous due to their longer lifespan and reduced maintenance requirements compared to other batteries. Businesses can benefit from lower per-unit costs and decreased long-term expenditures on battery replacement and maintenance. This economic efficiency is particularly beneficial for industries where batteries are a critical component of operational infrastructure, such as in renewable energy systems or electric mobility.
Performance Advantages
LiFePO4 batteries are known for their high energy density and low self-discharge rates, which translate to enhanced performance and reliability. Their ability to deliver high currents on demand makes them ideal for applications requiring robust power outputs, such as backup power systems and electric vehicles. Additionally, their performance consistency across varying temperatures and conditions ensures optimal functionality without the need for frequent monitoring or adjustments.
Environmental Benefits
The eco-friendly nature of LiFePO4 batteries is a significant draw for companies aiming to improve their environmental impact. By choosing LiFePO4 batteries, businesses not only comply with stringent environmental regulations but also contribute to a sustainable future. This commitment to environmental responsibility can enhance a company’s brand reputation and appeal to a growing demographic of eco-conscious consumers.
Adaptability
LiFePO4 batteries cater to a wide array of industries due to their versatile properties. Whether for grid energy storage, portable power solutions, or electric transportation, these batteries provide reliable and efficient energy solutions that adapt to various technological and operational demands. This adaptability makes LiFePO4 an excellent investment for businesses looking to future-proof their energy needs.
Demand for LiFePO4 Batteries in the US and European Markets
The demand for LiFePO4 batteries in the US and European markets is fueled by a combination of regulatory pressures, technological advancements, and shifting consumer preferences towards sustainability. These regions are at the forefront of implementing green initiatives and policies that incentivize the adoption of cleaner and more efficient technologies. LiFePO4 batteries, with their high efficiency and low environmental impact, align perfectly with these initiatives, making them increasingly popular in applications ranging from residential solar power systems to commercial electric vehicle fleets. The growing focus on reducing carbon footprints and enhancing energy independence further drives the demand for reliable and sustainable battery solutions like LiFePO4.
Selecting the Right LiFePO4 Battery Supplier
Key Considerations
Choosing the right supplier for LiFePO4 batteries is crucial for ensuring product quality, reliability, and value. Businesses should look for suppliers that not only offer competitive pricing but also provide comprehensive support services, including warranty provisions, customer service, and technical assistance. The ability of the supplier to consistently deliver high-quality products on time is also critical, especially in industries where delays can lead to significant operational disruptions. Additionally, the supplier’s commitment to environmental standards and their capability to offer customized battery solutions should be considered to ensure alignment with the purchasing company’s values and specific needs.
Conclusion
Reflecting on the various benefits and the growing market demand, it’s evident that LiFePO4 batteries represent a strategic investment for businesses looking to advance their technology base and improve their sustainability practices. The long-term cost savings, coupled with the performance reliability and environmental benefits, make LiFePO4 batteries an indispensable component of modern energy solutions. Emphasizing these points, businesses can make informed decisions that align with their operational goals and environmental commitments.

About Himax Electronics
Himax Electronics has established itself as a leader in the battery industry by consistently providing high-quality LiFePO4 batteries and tailored energy solutions that meet the specific needs of businesses. With a strong emphasis on innovation and sustainability, Himax Electronics supports its clients in achieving their operational objectives while contributing to a more sustainable future. Our commitment to excellence and customer satisfaction ensures that our clients receive the best possible products and services, supporting their journey towards greater energy efficiency and reduced environmental impact.