An example of modern technology implemented in the nautical vessel. Darling Harbor marina, Sydney, Australia.
It’s time. Your RV or boat’s lead-acid battery bank had a good run, but just isn’t able to hold a charge anymore – so what should you do? Using desulphators could help squeeze some more life out of it, but instead of asking how to restore lead-acid batteries that are clearly past their prime, the question you should be asking is: Can I replace lead-acid batteries with lithium batteries in my boat or RV? After all, lithium batteries are becoming the standard for renewable energy storage.
The answer is YES, you can absolutely replace lead-acid batteries with lithium in marine and RV applications – but here are a few considerations to help you decide if upgrading to lithium batteries is the right lead acid battery alternative for your boat, camper, or RV.
WHY REPLACE LEAD ACID BATTERIES WITH LITHIUM IN A BOAT OR RV?
Lead Acid vs. Lithium: Depth of Discharge
Depth of Discharge, or DoD, is how much of your battery bank’s stored energy can actually be used without dramatically reducing its life. For example, a 100Ah (amp hour) lead-acid battery rated for 25% DoD means you need to plan to use only ¼ of its rated capacity (so 25Ah), leaving the other ¾ in the battery, unused.
- DoD for lead-acid batteries – both flooded (which you have to add water to periodically) and sealed (“maintenance-free”) – is typically in the 25% – 50% range. Your battery will last at least twice as long if you regularly discharge it 25% than if you regularly discharge it 50%. Keep in mind that if you don’t have a sunny day to recharge your batteries after a day of use, the DoD will go down again the next day – so planning to use 25% per day will allow you to use less than the 50% maximum after two days of use.
- On the other hand, DoD for lithium ion batteries is 80% or more, allowing you to use most or even all of the battery’s stored energy. That means a 100Ah lithium battery rated for 80% DoD can safely provide you with 80Ah without being harmed.
As a result, a lithium battery bank can be much smaller than a lead-acid battery bank to provide the same amount of usable energy. For example, if you need 100Ah of energy a day, you would need a 400Ah lead-acid battery bank to stay at 25% DoD, but would only need 125Ah of lithium at 80% DoD. That is a significantly smaller battery bank with lithium batteries.
Lead Acid vs. Lithium: Cycle Count
Cycling a battery means discharging it to any amount and recharging it to a fully charged state. If you cycle your battery bank every day for a year, that’s 365 cycles. If you only use it on the weekends, and keep the bank topped off the rest of the time, that’s 104 cycles a year.
A cycle is a cycle regardless of how deep the discharge is, but the depth of discharge directly affects how many cycles you can expect your battery to last. A battery’s specs will tell you how many cycles to expect from it when discharging to its rated DoD.
- A standard flooded lead-acid battery can have about 2500 cycles at 25% DoD
- A standard sealed lead acid battery can have about 1200 cycles at 25% DoD
- Unlike lead-acid, lithium batteries don’t have a cycle curve under 80% DoD. Beyond 80%, the cycle count can drop dramatically. A typical lithium battery can have 5000+ cycles at up to 80% DoD. That’s 4x the cycles at over 3x the DoD. That’s a much longer lived battery bank with lithium batteries.
Lead Acid vs. Lithium: Charge/Discharge Rate
In addition to how much of a battery’s capacity you use, it also matters how fast you use it. Again using the 100Ah battery example, if you have a 10 amp (A) load, that can drain the battery completely in 10 hours (100Ah ÷ 10A = 10 hours). That is considered a C/10 rate. Likewise, if you have a 50A load on the same battery, that would drain it in 2 hours (100Ah ÷ 50A = 2 hours). That is a C/2 rate. Most batteries are rated at their C/20 rate, emptying the battery in 20 hours.
If you have a high-current load in your system, or are charging it very quickly with a high current, such as your alternator or shore power, you need to consider the charge/discharge rate of the battery bank. If you need a higher rate than the batteries can handle, you would need to increase the battery bank by adding more batteries in parallel so that the batteries can share the current between themselves. This may result in needing a battery bank that has a higher Ah capacity than you need to power your loads, just to handle the high current.
Likewise, too slow of a charge of lead-acid batteries can cause premature sulphation, shortening their life. This is not a problem with lithium.
- Lead-acid batteries tend to perform best between C/8 and C/12 rates. So our 100Ah battery would want to be charged or discharged at between 8A and 12A. Wiring three batteries in parallel would permit three times the rate, as it shares the current between the three, so 24A to 36A.
- Some lithium batteries can generally handle a C/1 rate, or even higher for short periods depending on the battery. This means a 100Ah lithium battery can handle 100A (or more) of charge/discharge current. Most manufacturers recommend no more than a C/2 rate on a regular basis for best battery life, but it is good to know the extra power is there with lithium batteries if you need it. Be sure to check the manufacturer’s specs when selecting a lithium battery, as some do not support as high of a current as others.
Lead Acid vs. Lithium: Voltage Sag
You may be familiar with the voltage of your boat or RV’s battery bank sagging, or dropping to 11V or lower when trying to run a high-power load such as your winch, windlass, or air conditioner. When running a heavy AC load off the inverter, the voltage could drop below the low voltage cutoff, causing the inverter to turn off when you need it most. Likewise, if you are running a DC load like your bow thruster directly off the battery bank, you need it to maintain a high enough voltage for it to work when you really need it to work. Due to lithium batteries’ voltage curve and ability to handle high current, loads like these will not cause the voltage to drop dramatically, eliminating the problem of voltage sag.
Lead Acid vs. Lithium: Size and Weight
With a higher DoD, higher cycle count, and higher charge/discharge rate, it’s easy to see how using lithium batteries in your RV or boat saves space by requiring a physically smaller battery bank…and I don’t need to explain the advantages of saving space in an already tight spot. But there’s yet another physical benefit of replacing lead-acid batteries with lithium for RV and marine applications: Lithium batteries also don’t have the crazy weight from being made with lead! Lighter weight means higher fuel efficiency, saving you additional money in gas or diesel costs.
Lead Acid vs. Lithium: Safety
Safety is always a primary consideration when designing a solar system, but it becomes even more important when your system is on a boat far from shore, or an RV on a remote road. Different battery chemistries have different risk factors. Obviously, abusing any type of battery can create a dangerous situation. But with normal, and perhaps even a bit of rough treatment, the different batteries have different safety concerns that need to be addressed.
- Flooded lead-acid batteries have an acid and water electrolyte in the battery that has to be checked on a regular basis. During normal charging cycles, this mixture turns into a gas that needs to be vented outside. A buildup of the gas inside a vehicle or vessel can be explosive. Proper ventilation mitigates this concern. The outgassing of the battery is normal, but requires owners to regularly check to see when the electrolyte level gets low from the outgassing. If low, it needs to have more distilled water added. This runs the risk of acid spills if overfilled or overcharged. This requires you to be prepared with proper safety equipment including gloves, safety glasses, and baking soda to neutralize the acid if needed.
- Sealed lead-acid batteries do not have outgassing or electrolyte levels to check, as they do not outgas. Normal battery safety measures should be followed, like checking for tight cable connections, corrosion, and preventing physical damage to the battery itself.
- Lithium batteries also do not outgas, but certain types (the ones with cobalt, known as lithium cobalt oxide or LCO) can experience thermal runaway – a condition where the battery starts to get hot, which causes it to react to the heat and get hotter and hotter until it catches on fire. LCO batteries are most commonly used in cell phones, hoverboards, and electric cars, and are generally not recommended for mobile applications.
So are lithium batteries any safer than other batteries? Yes – when they don’t contain cobalt. Lithium ferrous phosphate (LFP or LiFePO4) chemistry has become the standard lithium battery for marine, RV, and general solar PV use because they have no thermal runaway issues. They are very safe, can be installed indoors, and are a perfect solution for mobile living and recreation. Just as with sealed lead-acid batteries, making sure the cables haven’t shaken loose with vibration from travel, and a visual inspection to ensure all is well is all that is needed.
Lead Acid vs. Lithium: Temperature

Lead Acid Temperature Deration
Temperature has different impacts on different types of batteries. A lead-acid battery’s capacity is rated at 80°F (26°C), but the colder it gets, the more capacity falls. So our 100Ah lead-acid battery at 80°F holds only 76Ah at 40°F (4°C). As a result, if you know you are going to be using your battery bank in the winter, and they will be in an unconditioned location (not heated or cooled), you need to oversize your battery bank to make up for the smaller capacity when cold.
Lead-acid batteries also perform best when charged at different rates based on temperature. As a result, most quality solar charge controllers have a battery temperature sensor to report back to the charge controller.
Lithium batteries maintain the same capacity regardless of the temperature, and do not need their charging rate adjusted to account for temperature.
However, while you can run your loads in freezing temperatures, you cannot charge a lithium battery in sub-freezing temperatures (below 32°F or 0°C). A Battery Monitoring System (BMS) will often have cold temperature cut-off, preventing the battery from being charged when it is too cold.

If your lithium battery bank is in a cold environment, you need to either get a battery that has a built-in heater like the Himax battery, and/or build an insulated battery box to hold in the heat generated while charging.
Lead Acid vs. Lithium: Lifetime Cost
If you compare lithium batteries to lead-acid batteries Ah to Ah, lithium batteries are more expensive. But step back and look at the bigger picture – taking into account everything we’ve covered so far – and you can see how lithium batteries can actually save you money, time, and hassle in the long run.
Let’s look at some cost examples when designing a battery bank for a 12V system that uses 1400Wh a day.
Sealed AGM vs. Lithium
For a lithium bank: 1400Wh x 2 days of autonomy ÷ 80% DoD (after 2 days without sun, daily is 40%) ÷ 12V battery bank = 290Ah battery. I’ll round up to 300Ah and use two 1800 at $1300 each for a total of $2600.For a sealed AGM battery bank: 1400Wh x 2 days of autonomy (days without sun) x 1.11 temperature derate (60F) ÷ 50% DoD (25% x 2 days without sun) ÷ 12V battery bank = 518Ah bank. I’ll round up to 600Ah and use 200Ah 12V batteries at $600 each for a total of $1800.
At first

Flooded Lead Acid vs. Lithium
glance, the lithium bank costs more than the AGM bank. But when you consider cycle counts (1200 for the AGM and 5000 for the lithium), the lithium battery bank will last 4x longer than the AGM bank. You would need to buy four AGM battery banks for $7200 – and spend the time shopping for and installing them – to match the lifetime of one lithium bank for $2600. Plus you’d miss out on all the previously mentioned benefits of lithium batteries for a boat or RV.
The math is the same for a flooded lead-acid battery bank as for a sealed one. So let’s again compare a 518Ah 12V lead-acid battery bank with the 300Ah 12V lithium bank. I’ll round up to 675Ah to use the popular Trojan T-105 225Ah 6V batteries at $175 each. Using 6V batteries will require 2 in series to get 12V, so I’ll need 6 for a total of $1050. We are still going to use two of the KiloVault HLX1800 for $2600, or I could use a single 300Ah 12V battery for the $2500. Some people prefer installing two batteries in parallel for redundancy and find that the size and weight of two batteries may be easier to manage than one.
The price gap between flooded lead-acid and lithium is greater than with AGM. With flooded’s 2500 cycles versus lithium’s 5000 cycles, a well maintained flooded battery bank can last half as long as lithium. But a poorly maintained flooded battery bank can quickly become a boat anchor in a year or two. So the flooded is a slightly less expensive solution than sealed lead-acid at $2100 for two banks vs. $2600 for one lithium. But again, you have the advantages of the smaller, lighter, safer battery bank, the higher current capability, and minimal maintenance needed on the lithium. It may be worth the extra $500 to you to go lithium.
Lead Acid vs. Lithium for Marine and RVs: The Verdict
By choosing lithium batteries as a lead-acid battery alternative for marine/RV applications, you will need fewer batteries, and those batteries will last longer, cycle deeper, deliver more power, and weigh less.
CONSIDERATIONS WHEN REPLACING LEAD ACID BATTERIES WITH LITHIUM IN A BOAT OR RV
Now that you’re convinced lithium is the best way to go, you need to be aware of a few things when replacing a lead-acid battery with lithium. The term “drop-in replacement” has become popular, but the reality is there are a few other things you’ll need to do to safely upgrade from lead-acid to lithium batteries in your boat or RV.
Charge Controller/Charging Profile
If you are currently charging your lead acid batteries with solar, your alternator, and/or shore power, you may be able to keep your existing charge controller or inverter/charger. The charging and low voltage cutoff profiles for lithium batteries are a little different from lead-acid, so you need chargers that have adjustable charge rates. Different batteries will have different preferences, so be sure to see the manufacturer’s recommendations when configuring your charger. They will often recommend a Bulk and Absorb rate of around 14V, with an Absorption time of as little as 2 minutes, significantly less than the standard for lead-acid. With a Float voltage of just below 14V, you can maintain the charge without overcharging it. Because lithium has a very narrow voltage window, 12V is generally the lowest voltage you want before you shut off your loads.
Note: Unlike lead-acid batteries, lithium batteries do not always need to be recharged to their full 100% capacity. They actually prefer being in a partial state of charge. If you are going to be leaving your boat or RV for a season of storage, it is recommended that you leave the battery bank at around 90% state of charge. This leaves plenty of energy for small loads like the bilge pump or CO2 alarm, but helps maintain a healthy battery bank until you can get back to normal use.
Cranking Amps / Starter Battery
With lead-acid batteries, we are used to seeing a rating of CCA (cold crank amps) to show how many amps can be used to start an engine in the cold weather. Lithium batteries do not have the CCA rating. If you intend to replace a lead-acid battery with lithium for your starting battery, make sure the new lithium battery is rated to handle enough current to do so. Not all of them are. We see a lot of people continue to use a lead-acid battery as the starter, with lithium used only for the house/service battery. This also gives you a bit of a backup, so that if everything goes wrong with your house/service battery, you still have the starter battery available.
Alternator
Unlike lead-acid batteries, lithium batteries have very little internal resistance and can take as much charging current from the alternator as needed. But since alternators are not designed to run at full speed for long periods, this can result in the alternator working too hard, overheating, and damaging itself. There are a few ways to prevent this from happening.
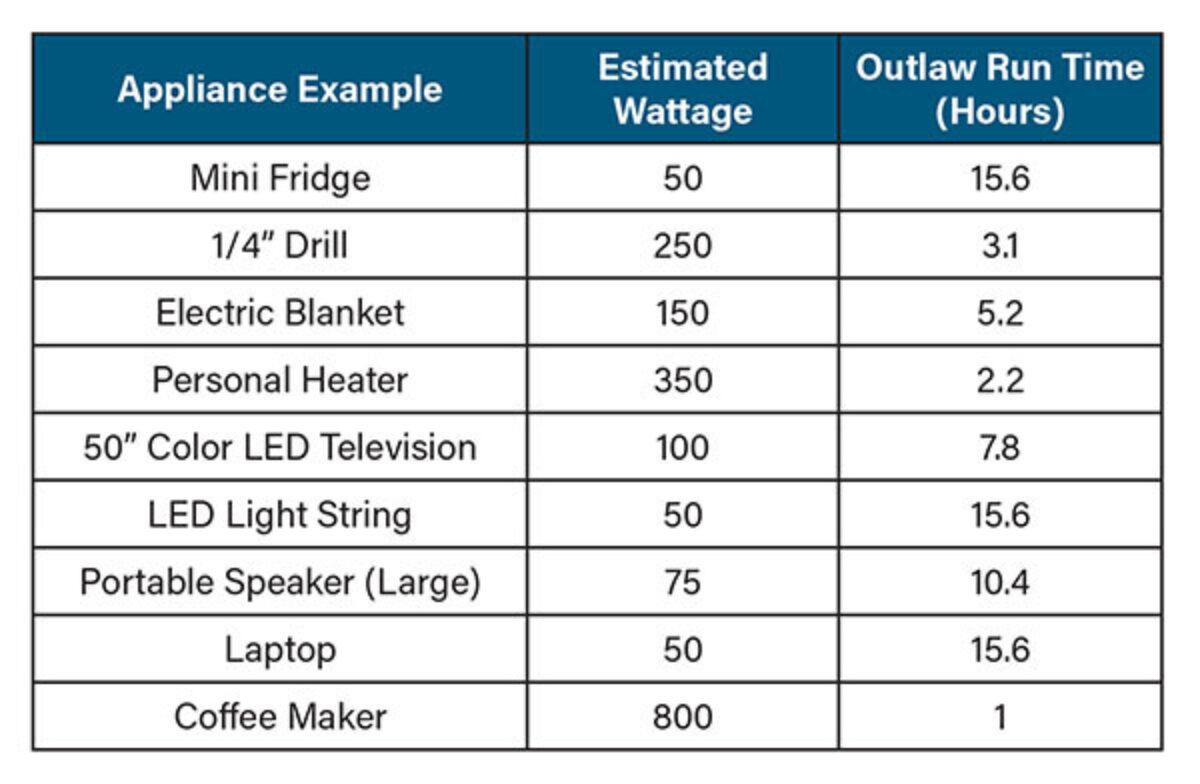
Use a DC/DC Converter
By installing a DC-to-DC converter between the alternator and the lithium battery bank, you can limit the amount of current the battery draws from the alternator. It is recommended that you only draw from the alternator at half its rating, so for a 60A alternator, a 30A DC/DC converter like the Bluetooth-enabled Victron Energy Orion-Tr Smart 12/12-30A charger is a good option. You can use multiple DC/DC converters in parallel to increase the rate for larger alternators.
Victron Orion-TR Smart DC/DC Converter setup
Replace the Alternator
You can replace the alternator with one designed for higher amperage charging and temperature control. Balmar makes great alternators and external regulators for this. They monitor the temperature and will wind down to appropriate amperage if the alternator gets too hot. If you currently have a V-belt, you may need to modify the engine for a serpentine belt before you can use the larger Balmar alternator.
Low Voltage Disconnect
The ability to automatically disconnect your DC loads gives you control over how low you discharge your battery bank. An automatic switch such as the Victron Energy Smart BatteryProtect can be configured via Bluetooth for excellent control of your system. It can turn your non-critical loads on or off based on a configurable voltage setting.
Battery/Bank Monitoring
KiloVault CHLX Bluetooth App
Any good battery system should have the ability to monitor both the individual batteries, and the whole battery bank. Watching more than just the voltage, but also how many amps go in and out of the battery bank and the temperature gives you a complete view of the health and state of charge of the entire bank. Some lithium battery Battery Monitoring Systems have Bluetooth or WiFi built in to allow you to monitor it from the phone. For example, the KiloVault HLX and CHLX batteries have Bluetooth to your Smartphone to see down to the cell level of each battery.
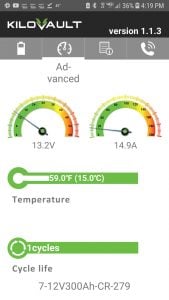
The Victron Energy Smart Shunt provides a low cost method to monitor your whole battery bank via Bluetooth from your smartphone. It does not include a display, so you can only view it via Bluetooth.
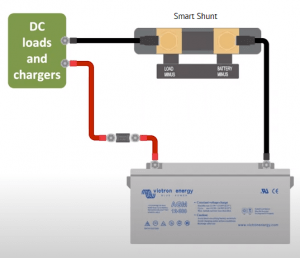
Victron SmartShunt – Monitors Batteries via Bluetooth
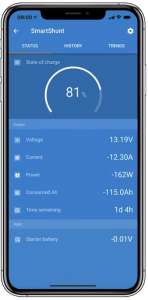
The Victron Energy Smart Battery Monitor BVM-712 gives you a local display for convenient viewing of battery voltage, current, power, amp-hours consumed, and state of charge (SoC). It can also be viewed via BlueTooth.

IT’S TIME TO MOBILIZE WITH LITHIUM BATTERIES FOR MARINE AND RV APPLICATIONS
Whether you are looking for a new battery bank for your RV or boat or considering replacing your aging lead-acid batteries, deep-cycle lithium-ion batteries – specifically LiFePO4 batteries – are an excellent solution. Compared to lead-acid batteries, LiFePO4 batteries offer more power, higher current, a longer life, smaller footprint, lower weight, and safe, maintenance-free operation. Are you ready to mobilize and go lithium?
See more options for lithium batteries at our website or contact us at (86)755-2562 9920 to help you select the right lithium batteries for your specific needs.