How to Build a LiFePO4 Battery: A Complete Guide
Building a LiFePO4 (Lithium Iron Phosphate) battery from scratch is a rewarding project for anyone interested in renewable energy technology, DIY electronics, or advanced battery systems. LiFePO4 batteries offer several advantages over traditional lithium-ion products, including greater thermal stability, higher safety margins, and longer life cycles. This detailed guide will walk you through the steps to build your own LiFePO4 battery, highlighting the role of Himax Electronics in optimizing your battery build.
Understanding LiFePO4 Batteries
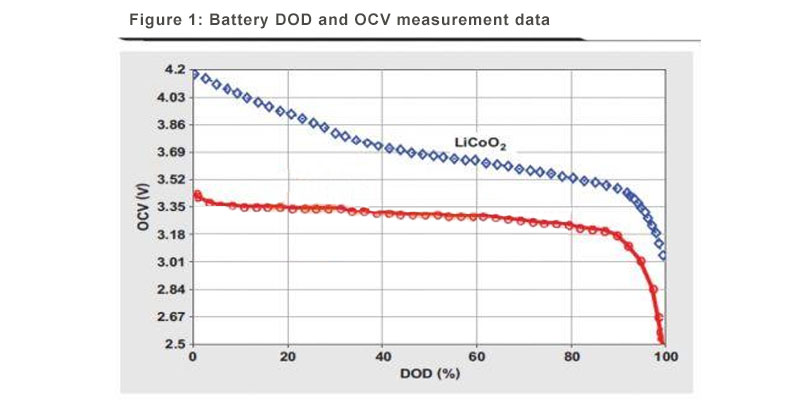
LiFePO4 batteries are popular for applications requiring high load currents and endurance, making them perfect for electric vehicles, solar energy storage, and portable power stations. They are less prone to thermal runaway than other lithium batteries, offering a safer alternative for DIY projects.
Materials Needed
- LiFePO4 Cells: Choose cells based on your required voltage and capacity.
- Battery Management System (BMS): Essential for protecting the battery against overcharge, deep discharge, and cell imbalance.
- Connectors: High-quality connectors suitable for high current.
- Cables and Wiring: Appropriate gauge wires to handle the expected current.
- Soldering Iron and Supplies: For making secure connections.
- Enclosure: To safely house all components.
- Thermal Management Materials: Like heat sinks or cooling fans, depending on your application.
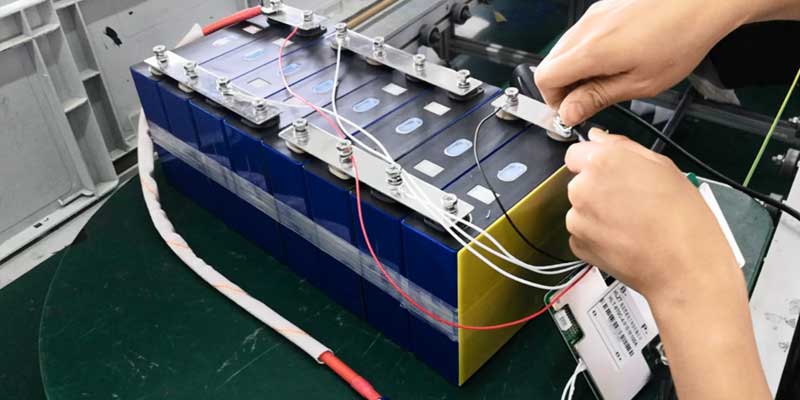
Steps to Build a LiFePO4 Battery
- Design the Battery Pack:
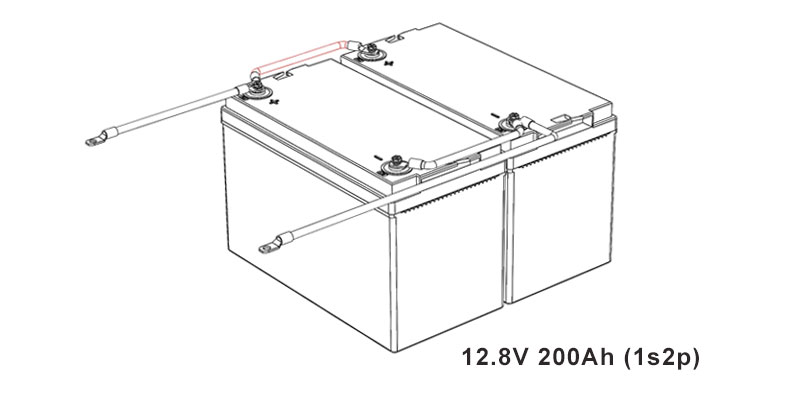
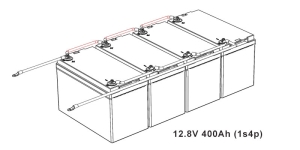
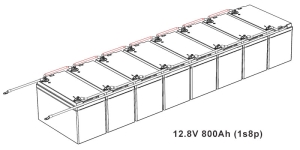
- Determine the Configuration: Calculate the total voltage and capacity you need. For example, to make a 12V battery, you would series connect four 3.2V cells.
- Plan Capacity: Decide how many cells you need in parallel to achieve the desired amp-hour rating.
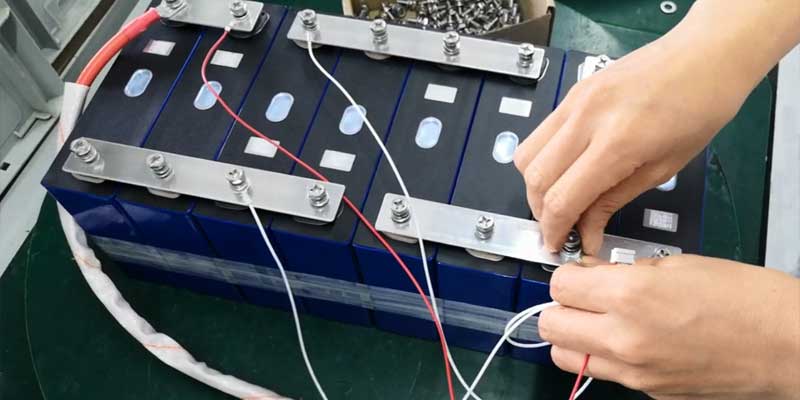
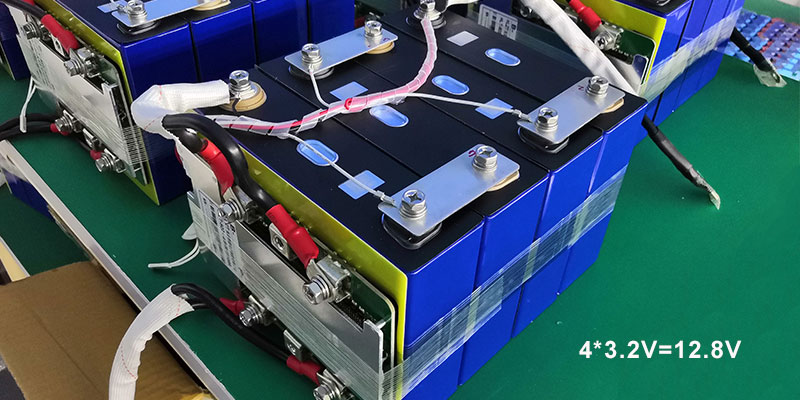
- Assemble the Cells:
- Series Connection: Connect the positive terminal of one cell to the negative of the next to add up their voltages.
- Parallel Connection: Connect the positive terminals together and the negatives together to increase capacity without increasing voltage.
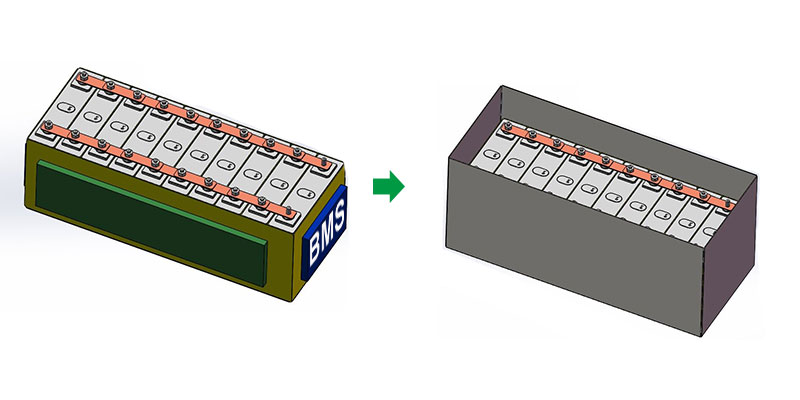
- Install the BMS:
- Connect the BMS: Attach the BMS to all cell terminals to monitor and balance each cell’s voltage, ensuring safe charging and discharging.
- Wiring and Connections:
- Secure Connections: Use the soldering iron to securely attach cables to each terminal, ensuring robust connections that can handle the battery’s current.
- Insulate Exposed Wires: Use heat shrink tubing or electrical tape to cover all exposed wires and terminals, preventing shorts.
- Enclosure Setup:
- Mount the Cells: Securely mount the cells within a non-conductive, durable enclosure.
- Install Thermal Management: Depending on your setup, incorporate cooling systems to manage heat during operation.
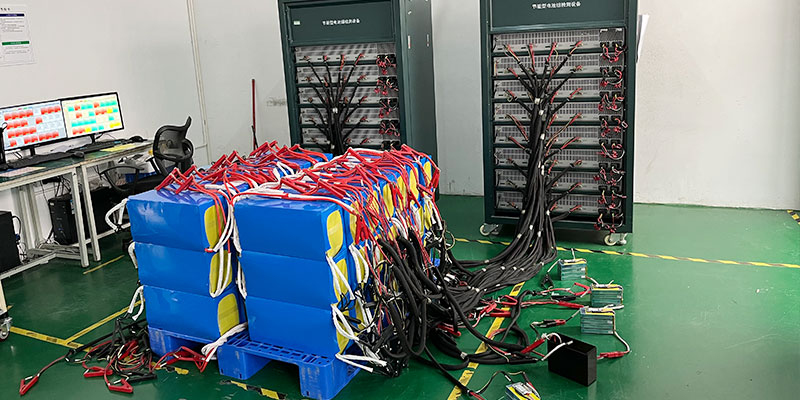
- Testing and Activation:
- Initial Charging: Slowly charge the battery under supervision to make sure all cells are balancing correctly.
- Load Testing: Test the battery under operational loads to ensure it meets expected specifications.

Integrating Himax Electronics
Himax Electronics offers critical components and expertise that can greatly enhance the process of building a LiFePO4 battery:
- Advanced BMS Options: Himax provides sophisticated BMS systems tailored to various setups, ensuring longevity and safety.
- Custom Cells and Modules: For specific projects, Himax can supply custom cells and modules that fit unique requirements, backed by industry-leading technology.
- Technical Support: Himax’s expert team offers support in system design, installation, and optimization, providing you with insights and solutions to build an efficient and reliable battery system.
Conclusion
Building a LiFePO4 battery requires careful planning and precise execution but can result in a highly efficient and robust power source. Whether you’re creating a backup power system, a portable energy solution, or integrating it into a renewable setup, the principles remain the same. Himax Electronics is ready to support your projects with high-quality components and expert guidance.
For more information or to consult with a battery expert, visit Himax Electronics’ website or contact their support team directly. They can provide further details on components, customization options, and more to help ensure your battery build is a success.