What’s the Best Battery?
What’s the Best Battery?
We often get puzzled by announcements of new batteries that are said to offer very high energy densities, deliver 1000 charge/discharge cycle and are paper-thin. Are they real? Perhaps — but not in one and the same battery. While one battery type may be designed for small size and long runtime, this pack will not last and wear out prematurely. Another battery may be built for long life, but the size is big and bulky. A third battery may provide all the desirable attributes, but the price would be too high for commercial use.
Battery manufacturers are well aware of customer needs and have responded by offering packs that best suit the specific applications. The mobile phone industry is an example of clever adaptation. Emphasis is placed on small size, high energy density and low price. Longevity comes in second.
The inscription of NiMH on a battery pack does not automatically guarantee high energy density. A prismatic Nickel-Metal Hydride battery for a mobile phone, for example, is made for slim geometry. Such a pack provides an energy density of about 60Wh/kg and the cycle count is around 300. In comparison, a cylindrical NiMH offers energy densities of 80Wh/kg and higher. Still, the cycle count of this battery is moderate to low. High durability NiMH batteries, which endure 1000 discharges, are commonly packaged in bulky cylindrical cells. The energy density of these cells is a modest 70Wh/kg.
Compromises also exist on lithium-based batteries. Li-ion packs are being produced for defense applications that far exceed the energy density of the commercial equivalent. Unfortunately, these super-high capacity Li-ion batteries are deemed unsafe in the hands of the public and the high price puts them out of reach of the commercial market.
In this article we look at the advantages and limitations of the commercial battery. The so-called miracle battery that merely live in controlled environments is excluded. We scrutinize the batteries not only in terms of energy density but also longevity, load characteristics, maintenance requirements, self-discharge and operational costs. Since NiCd remains a standard against which other batteries are compared, we evaluate alternative chemistries against this classic battery type.
Nickel Cadmium (NiCd) — mature and well understood but relatively low in energy density. The NiCd is used where long life, high discharge rate and economical price are important. Main applications are two-way radios, biomedical equipment, professional video cameras and power tools. The NiCd contains toxic metals and is environmentally unfriendly.
Nickel-Metal Hydride (NiMH) — has a higher energy density compared to the NiCd at the expense of reduced cycle life. NiMH contains no toxic metals. Applications include mobile phones and laptop computers.
Lead Acid — most economical for larger power applications where weight is of little concern. The lead acid battery is the preferred choice for hospital equipment, wheelchairs, emergency lighting and UPS systems.
Lithium Ion (Li-ion) — fastest growing battery system. Li-ion is used where high-energy density and lightweight is of prime importance. The technology is fragile and a protection circuit is required to assure safety. Applications include notebook computers and cellular phones.
Lithium Ion Polymer (Li-ion polymer) — offers the attributes of the Li-ion in ultra-slim geometry and simplified packaging. Main applications are mobile phones.
Figure 1 compares the characteristics of the six most commonly used rechargeable battery systems in terms of energy density, cycle life, exercise requirements and cost. The figures are based on average ratings of commercially available batteries at the time of publication.
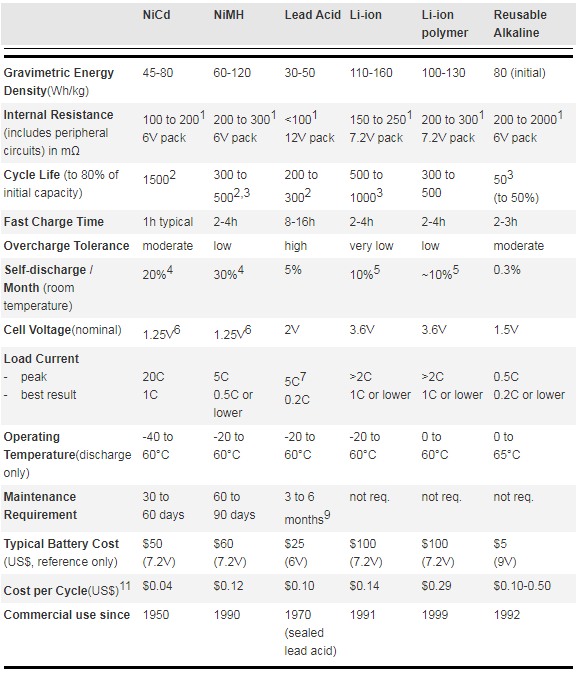
Figure 1: Characteristics of commonly used rechargeable batteries
- Internal resistance of a battery pack varies with cell rating, type of protection circuit and number of cells. Protection circuit of Li-ion and Li-polymer adds about 100mΩ.
- Cycle life is based on battery receiving regular maintenance. Failing to apply periodic full discharge cycles may reduce the cycle life by a factor of three.
- Cycle life is based on the depth of discharge. Shallow discharges provide more cycles than deep discharges.
- The discharge is highest immediately after charge, then tapers off. The NiCd capacity decreases 10% in the first 24h, then declines to about 10% every 30 days thereafter. Self-discharge increases with higher temperature.
- Internal protection circuits typically consume 3% of the stored energy per month.
- 25V is the open cell voltage. 1.2V is the commonly used value. There is no difference between the cells; it is simply a method of rating.
- Capable of high current pulses.
- Applies to discharge only; charge temperature range is more confined.
- Maintenance may be in the form of ‘equalizing’ or ‘topping’ charge.
- Cost of battery for commercially available portable devices.
- Derived from the battery price divided by cycle life. Does not include the cost of electricity and chargers.
Observation: It is interesting to note that NiCd has the shortest charge time, delivers the highest load current and offers the lowest overall cost-per-cycle, but has the most demanding maintenance requirements.
The Nickel Cadmium (NiCd) battery
The NiCd prefers fast charge to slow charge and pulse charge to DC charge. All other chemistries prefer a shallow discharge and moderate load currents. The NiCd is a strong and silent worker; hard labor poses no problem. In fact, the NiCd is the only battery type that performs well under rigorous working conditions. It does not like to be pampered by sitting in chargers for days and being used only occasionally for brief periods. A periodic full discharge is so important that, if omitted, large crystals will form on the cell plates (also referred to as memory) and the NiCd will gradually lose its performance.
Among rechargeable batteries, NiCd remains a popular choice for applications such as two-way radios, emergency medical equipment and power tools. Batteries with higher energy densities and less toxic metals are causing a diversion from NiCd to newer technologies.
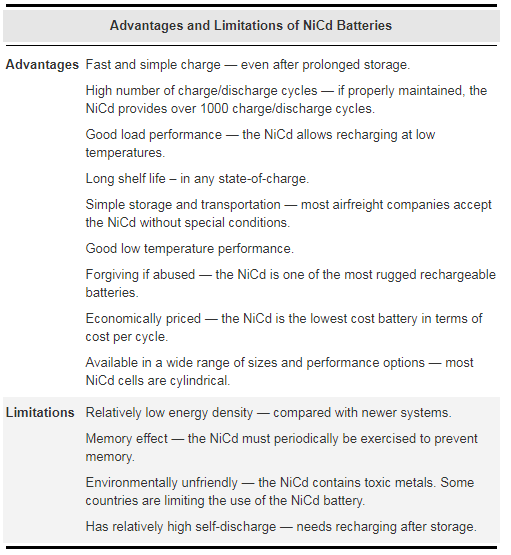
Figure 2: Advantages and limitations of NiCd batteries.
The Nickel-Metal Hydride (NiMH) battery
Research of the NiMH system started in the 1970s as a means of discovering how to store hydrogen for the nickel hydrogen battery. Today, nickel hydrogen batteries are mainly used for satellite applications. They are bulky, contain high-pressure steel canisters and cost thousands of dollars per cell.
In the early experimental days of the NiMH battery, the metal hydride alloys were unstable in the cell environment and the desired performance characteristics could not be achieved. As a result, the development of the NiMH slowed down. New hydride alloys were developed in the 1980s that were stable enough for use in a cell. Since the late 1980s, NiMH has steadily improved.
The success of the NiMH has been driven by its high energy density and the use of environmentally friendly metals. The modern NiMH offers up to 40 percent higher energy density compared to NiCd. There is potential for yet higher capacities, but not without some negative side effects.
The NiMH is less durable than the NiCd. Cycling under heavy load and storage at high temperature reduces the service life. The NiMH suffers from high self-discharge, which is considerably greater than that of the NiCd.
The NiMH has been replacing the NiCd in markets such as wireless communications and mobile computing. In many parts of the world, the buyer is encouraged to use NiMH rather than NiCd batteries. This is due to environmental concerns about careless disposal of the spent battery.
Experts agree that the NiMH has greatly improved over the years, but limitations remain. Most of the shortcomings are native to the nickel-based technology and are shared with the NiCd battery. It is widely accepted that NiMH is an interim step to lithium battery technology.
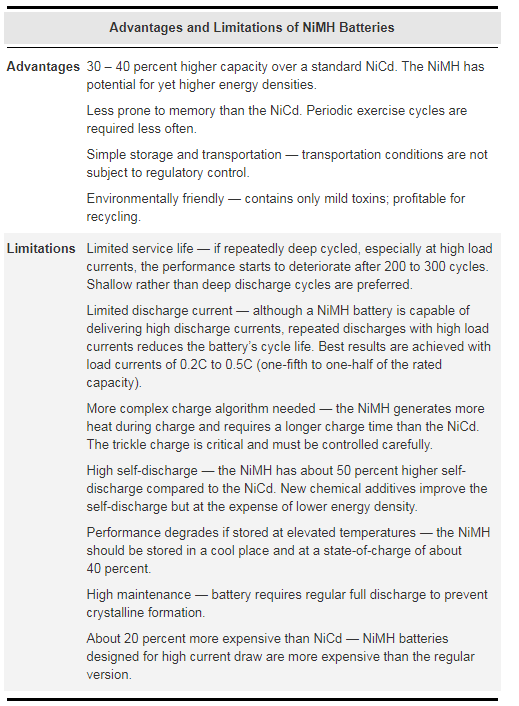
Figure 3: Advantages and limitations of NiMH batteries
The Lead Acid battery
Invented by the French physician Gaston Planté in 1859, lead acid was the first rechargeable battery for commercial use. Today, the flooded lead acid battery is used in automobiles, forklifts and large uninterruptible power supply (UPS) systems.
During the mid 1970s, researchers developed a maintenance-free lead acid battery that could operate in any position. The liquid electrolyte was transformed into moistened separators and the enclosure was sealed. Safety valves were added to allow venting of gas during charge and discharge.
Driven by different applications, two battery designations emerged. They are the small sealed lead acid (SLA), also known under the brand name of Gelcell, and the large valve regulated lead acid (VRLA). Technically, both batteries are the same. (Engineers may argue that the word ‘sealed lead acid’ is a misnomer because no lead acid battery can be totally sealed.) Because of our emphasis on portable batteries, we focus on the SLA.
Unlike the flooded lead acid battery, both the SLA and VRLA are designed with a low over-voltage potential to prohibit the battery from reaching its gas-generating potential during charge. Excess charging would cause gassing and water depletion. Consequently, these batteries can never be charged to their full potential.
The lead acid is not subject to memory. Leaving the battery on float charge for a prolonged time does not cause damage. The battery’s charge retention is best among rechargeable batteries. Whereas the NiCd self-discharges approximately 40 percent of its stored energy in three months, the SLA self-discharges the same amount in one year. The SLA is relatively inexpensive to purchase but the operational costs can be more expensive than the NiCd if full cycles are required on a repetitive basis.
The SLA does not lend itself to fast charging — typical charge times are 8 to 16 hours. The SLA must always be stored in a charged state. Leaving the battery in a discharged condition causes sulfation, a condition that makes the battery difficult, if not impossible, to recharge.
Unlike the NiCd, the SLA does not like deep cycling. A full discharge causes extra strain and each cycle robs the battery of a small amount of capacity. This wear-down characteristic also applies to other battery chemistries in varying degrees. To prevent the battery from being stressed through repetitive deep discharge, a larger SLA battery is recommended.
Depending on the depth of discharge and operating temperature, the SLA provides 200 to 300 discharge/ charge cycles. The primary reason for its relatively short cycle life is grid corrosion of the positive electrode, depletion of the active material and expansion of the positive plates. These changes are most prevalent at higher operating temperatures. Cycling does not prevent or reverse the trend.
The optimum operating temperature for the SLA and VRLA battery is 25°C (77°F). As a rule of thumb, every 8°C (15°F) rise in temperature will cut the battery life in half. VRLA that would last for 10 years at 25°C will only be good for 5 years if operated at 33°C (95°F). The same battery would endure a little more than one year at a temperature of 42°C (107°F).
Among modern rechargeable batteries, the lead acid battery family has the lowest energy density, making it unsuitable for handheld devices that demand compact size. In addition, performance at low temperatures is poor.
The SLA is rated at a 5-hour discharge or 0.2C. Some batteries are even rated at a slow 20-hour discharge. Longer discharge times produce higher capacity readings. The SLA performs well on high pulse currents. During these pulses, discharge rates well in excess of 1C can be drawn.
In terms of disposal, the SLA is less harmful than the NiCd battery but the high lead content makes the SLA environmentally unfriendly.
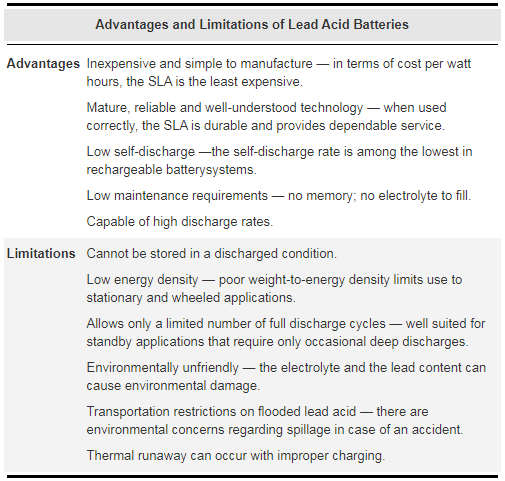
Figure 4: Advantages and limitations of lead acid batteries.
The Lithium Ion battery
Pioneer work with the lithium battery began in 1912 under G.N. Lewis but it was not until the early 1970s that the first non-rechargeable lithium batteries became commercially available. Lithium is the lightest of all metals, has the greatest electrochemical potential and provides the largest energy density per weight.
Attempts to develop rechargeable lithium batteries followed in the 1980s, but failed due to safety problems. Because of the inherent instability of lithium metal, especially during charging, research shifted to a non-metallic lithium battery using lithium ions. Although slightly lower in energy density than lithium metal, the Li-ion is safe, provided certain precautions are met when charging and discharging. In 1991, the Sony Corporation commercialized the first Li-ion battery. Other manufacturers followed suit. Today, the Li-ion is the fastest growing and most promising battery chemistry.
The energy density of the Li-ion is typically twice that of the standard NiCd. Improvements in electrode active materials have the potential of increasing the energy density close to three times that of the NiCd. In addition to high capacity, the load characteristics are reasonably good and behave similarly to the NiCd in terms of discharge characteristics (similar shape of discharge profile, but different voltage). The flat discharge curve offers effective utilization of the stored power in a desirable voltage spectrum.
The high cell voltage allows battery packs with only one cell. Most of today’s mobile phones run on a single cell, an advantage that simplifies battery design. To maintain the same power, higher currents are drawn. Low cell resistance is important to allow unrestricted current flow during load pulses.
The Li-ion is a low maintenance battery, an advantage that most other chemistries cannot claim. There is no memory and no scheduled cycling is required to prolong the battery’s life. In addition, the self-discharge is less than half compared to NiCd, making the Li-ion well suited for modern fuel gauge applications. Li-ion cells cause little harm when disposed.
Despite its overall advantages, Li-ion also has its drawbacks. It is fragile and requires a protection circuit to maintain safe operation. Built into each pack, the protection circuit limits the peak voltage of each cell during charge and prevents the cell voltage from dropping too low on discharge. In addition, the cell temperature is monitored to prevent temperature extremes. The maximum charge and discharge current is limited to between 1C and 2C. With these precautions in place, the possibility of metallic lithium plating occurring due to overcharge is virtually eliminated.
Aging is a concern with most Li-ion batteries and many manufacturers remain silent about this issue. Some capacity deterioration is noticeable after one year, whether the battery is in use or not. Over two or perhaps three years, the battery frequently fails. It should be noted that other chemistries also have age-related degenerative effects. This is especially true for the NiMH if exposed to high ambient temperatures.
Storing the battery in a cool place slows down the aging process of the Li-ion (and other chemistries). Manufacturers recommend storage temperatures of 15°C (59°F). In addition, the battery should be partially charged during storage.
Manufacturers are constantly improving the chemistry of the Li-ion battery. New and enhanced chemical combinations are introduced every six months or so. With such rapid progress, it is difficult to assess how well the revised battery will age.
The most economical Li-ion battery in terms of cost-to-energy ratio is the cylindrical 18650 cell. This cell is used for mobile computing and other applications that do not demand ultra-thin geometry. If a slimmer pack is required (thinner than 18 mm), the prismatic Li-ion cell is the best choice. There are no gains in energy density over the 18650, however, the cost of obtaining the same energy may double.
For ultra-slim geometry (less than 4 mm), the only choice is Li-ion polymer. This is the most expensive system in terms of cost-to-energy ratio. There are no gains in energy density and the durability is inferior to the rugged 18560 cell.
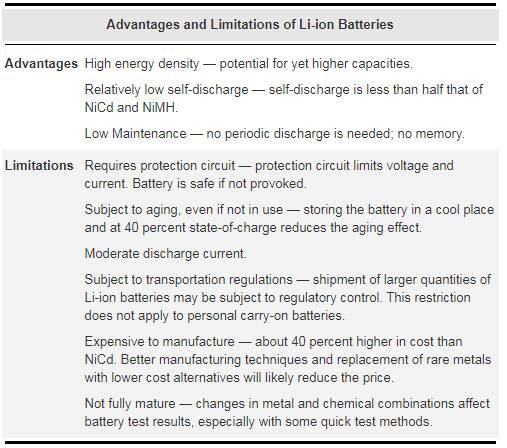
Figure 5: Advantages and limitations of Li-ion batteries
The Lithium Polymer battery
The Li-polymer differentiates itself from other battery systems in the type of electrolyte used. The original design, dating back to the 1970s, uses a dry solid polymer electrolyte. This electrolyte resembles a plastic-like film that does not conduct electricity but allows an exchange of ions (electrically charged atoms or groups of atoms). The polymer electrolyte replaces the traditional porous separator, which is soaked with electrolyte.
The dry polymer design offers simplifications with respect to fabrication, ruggedness, safety and thin-profile geometry. There is no danger of flammability because no liquid or gelled electrolyte is used. With a cell thickness measuring as little as one millimeter (0.039 inches), equipment designers are left to their own imagination in terms of form, shape and size.
Unfortunately, the dry Li-polymer suffers from poor conductivity. Internal resistance is too high and cannot deliver the current bursts needed for modern communication devices and spinning up the hard drives of mobile computing equipment. Heating the cell to 60°C (140°F) and higher increases the conductivity but this requirement is unsuitable for portable applications.
To make a small Li-polymer battery conductive, some gelled electrolyte has been added. Most of the commercial Li-polymer batteries used today for mobile phones are a hybrid and contain gelled electrolyte. The correct term for this system is Lithium Ion Polymer. For promotional reasons, most battery manufacturers mark the battery simply as Li-polymer. Since the hybrid lithium polymer is the only functioning polymer battery for portable use today, we will focus on this chemistry.
With gelled electrolyte added, what then is the difference between classic Li-ion and Li-ion polymer? Although the characteristics and performance of the two systems are very similar, the Li-ion polymer is unique in that solid electrolyte replaces the porous separator. The gelled electrolyte is simply added to enhance ion conductivity.
Technical difficulties and delays in volume manufacturing have deferred the introduction of the Li-ion polymer battery. In addition, the promised superiority of the Li-ion polymer has not yet been realized. No improvements in capacity gains are achieved — in fact, the capacity is slightly less than that of the standard Li-ion battery. For the present, there is no cost advantage. The major reason for switching to the Li-ion polymer is form factor. It allows wafer-thin geometries, a style that is demanded by the highly competitive mobile phone industry.




