Types of Lithium-Ion Batteries: Chemistries, Applications, and Future Trends
Become familiar with the many different types of lithium-ion batteries.
Lithium-ion (Li-ion) batteries have revolutionized modern energy storage, powering everything from smartphones and laptops to electric vehicles (EVs) and renewable energy systems. However, not all lithium batteries are created equal. Different chemistries—each with unique characteristics in terms of energy density, safety, lifespan, and cost—determine how a battery performs in real-world applications. This guide explores the main types of lithium-ion batteries, their advantages, limitations, and future developments.
Core Lithium-Ion Battery Chemistries
Lithium Cobalt Oxide (LCO)
- Composition: Lithium cobalt oxide cathode, graphite anode
- Key Traits: High energy density, relatively low cycle life, thermal stability issues
- Applications: Smartphones, laptops, tablets, cameras
- Limitations: Costly cobalt material, prone to overheating, limited lifespan
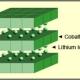
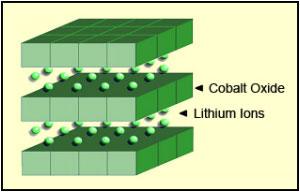 |
| Figure 1: Li-cobalt structure. The cathode has a layered structure. During discharge the lithium ions move from the anode to the cathode; on charge the flow is from cathode to anode. Source: Cadex |
| Lithium Cobalt Oxide: LiCoO2 cathode (~60% Co), graphite anode Short form: LCO or Li-cobalt.Since 1991 |
|
| Voltages | 3.60V nominal; typical operating range 3.0–4.2V/cell |
| Specific energy (capacity) | 150–200Wh/kg. Specialty cells provide up to 240Wh/kg. |
| Charge (C-rate) | 0.7–1C, charges to 4.20V (most cells); 3h charge typical. Charge current above 1C shortens battery life. |
| Discharge (C-rate) | 1C; 2.50V cut off. Discharge current above 1C shortens battery life. |
| Cycle life | 500–1000, related to depth of discharge, load, temperature |
| Thermal runaway | 150°C (302°F). Full charge promotes thermal runaway |
| Applications | Mobile phones, tablets, laptops, cameras |
| Comments
2019 update: |
Very high specific energy, limited specific power. Cobalt is expensive. Serves as Energy Cell. Market share has stabilized.
Early version; no longer relevant. |
Table 3: Characteristics of lithium cobalt oxide.
Lithium Manganese Oxide (LiMn2O4) — LMO
- Composition: Spinel-structured lithium manganese oxide
- Key Traits: High thermal stability, moderate energy density, safer than LCO
- Applications: Medical devices, power tools, early-generation EVs (Nissan Leaf)
- Limitations: Shorter lifespan compared to NMC and LFP
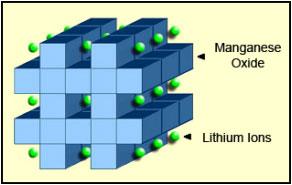 |
| Figure 4: Li-manganese structure. The cathode crystalline formation of lithium manganese oxide has a three-dimensional framework structure that appears after initial formation. Spinel provides low resistance but has a more moderate specific energy than cobalt. Source: Cadex |
Li-manganese has a capacity that is roughly one-third lower than Li-cobalt. Design flexibility allows engineers to maximize the battery for either optimal longevity (life span), maximum load current (specific power) or high capacity (specific energy). For example, the long-life version in the 18650 cell has a moderate capacity of only 1,100mAh; the high-capacity version is 1,500mAh.
Figure 5 shows the spider web of a typical Li-manganese battery. The characteristics appear marginal but newer designs have improved in terms of specific power, safety and life span. Pure Li-manganese batteries are no longer common today; they may only be used for special applications.
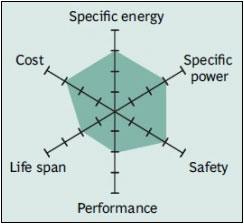 |
| Figure 5: Snapshot of a pure Li-manganese battery. Although moderate in overall performance, newer designs of Li-manganese offer improvements in specific power, safety and life span. Source: Boston Consulting Group |
Summary Table
| Lithium Manganese Oxide: LiMn2O4 cathode. graphite anode Short form: LMO or Li-manganese (spinel structure) Since 1996 |
|
| Voltages | 3.70V (3.80V) nominal; typical operating range 3.0–4.2V/cell |
| Specific energy (capacity) | 100–150Wh/kg |
| Charge (C-rate) | 0.7–1C typical, 3C maximum, charges to 4.20V (most cells) |
| Discharge (C-rate) | 1C; 10C possible with some cells, 30C pulse (5s), 2.50V cut-off |
| Cycle life | 300–700 (related to depth of discharge, temperature) |
| Thermal runaway | 250°C (482°F) typical. High charge promotes thermal runaway |
| Applications | Power tools, medical devices, electric powertrains |
| Comments
2019 update: |
High power but less capacity; safer than Li-cobalt; commonly mixed with NMC to improve performance.
Less relevant now; limited growth potential. |
Table 6: Characteristics of Lithium Manganese Oxide.
Lithium Nickel Manganese Cobalt Oxide (LiNiMnCoO2) — NMC
- Composition: Combination of nickel, manganese, and cobalt
- Key Traits: Excellent balance of energy density, power output, lifespan
- Applications: Electric vehicles, e-bikes, energy storage systems
- Limitations: Higher cost due to cobalt, sensitive to temperature
- Variants: NMC 111, 532, 622, and high-energy NMC 811 (low-cobalt trend)
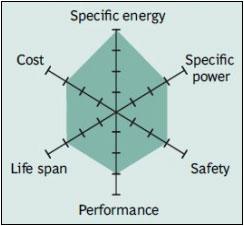 |
| Figure 7: Snapshot of NMC. NMC has good overall performance and excels on specific energy. This battery is the preferred candidate for the electric vehicle and has the lowest self-heating rate. Source: Boston Consulting Group |
There is a move towards NMC-blended Li-ion as the system can be built economically and it achieves a good performance. The three active materials of nickel, manganese and cobalt can easily be blended to suit a wide range of applications for automotive and energy storage systems (ESS) that need frequent cycling. The NMC family is growing in its diversity.
Summary Table
| Lithium Nickel Manganese Cobalt Oxide: LiNiMnCoO2. cathode, graphite anode Short form: NMC (NCM, CMN, CNM, MNC, MCN similar with different metal combinations) Since 2008 |
|
| Voltages | 3.60V, 3.70V nominal; typical operating range 3.0–4.2V/cell, or higher |
| Specific energy (capacity) | 150–220Wh/kg |
| Charge (C-rate) | 0.7–1C, charges to 4.20V, some go to 4.30V; 3h charge typical. Charge current above 1C shortens battery life. |
| Discharge (C-rate) | 1C; 2C possible on some cells; 2.50V cut-off |
| Cycle life | 1000–2000 (related to depth of discharge, temperature) |
| Thermal runaway | 210°C (410°F) typical. High charge promotes thermal runaway |
| Cost | ~$420 per kWh (Source: RWTH, Aachen) |
| Applications | E-bikes, medical devices, EVs, industrial |
| Comments
2019 update: |
Provides high capacity and high power. Serves as Hybrid Cell. Favorite chemistry for many uses; market share is increasing.
Leading system; dominant cathode chemistry. |
Table 8: Characteristics of lithium nickel manganese cobalt oxide (NMC).
Lithium Iron Phosphate(LiFePO4) — LFP
- Composition: Lithium iron phosphate cathode
- Key Traits: High safety, long cycle life, excellent thermal stability, lower energy density
- Applications: Electric buses, stationary storage, entry-level EVs, solar storage
- Limitations: Lower energy density than NMC and NCA, bulkier pack size
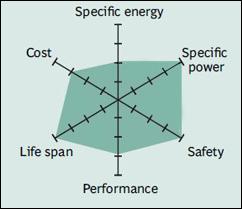 |
| Figure 9: Snapshot of a typical Li-phosphate battery. Li-phosphate has excellent safety and long life span but moderate specific energy and elevated self-discharge. Source: Cadex |
Summary Table
| Lithium Iron Phosphate: LiFePO4 cathode, graphite anode Short form: LFP or Li-phosphate. LIP is also common. Since 1996 |
|
| Voltages | 3.20, 3.30V nominal; typical operating range 2.5–3.65V/cell |
| Specific energy (capacity) | 90–120Wh/kg |
| Charge (C-rate) | 1C typical, charges to 3.65V; 3h charge time typical |
| Discharge (C-rate) | 1C, 25C on some cells; 40A pulse (2s); 2.50V cut-off (lower that 2V causes damage) |
| Cycle life | 2000 and higher (related to depth of discharge, temperature) |
| Thermal runaway | 270°C (518°F) Very safe battery even if fully charged |
| Cost | ~$580 per kWh (Source: RWTH, Aachen) |
| Applications | Portable and stationary needing high load currents and endurance |
| Comments
2019 update: |
Very flat voltage discharge curve but low capacity. One of safest Li-ions. Used for special markets. Elevated self-discharge.Used primarily for energy storage, moderate growth. |
Table 10: Characteristics of lithium iron phosphate.
Lithium Nickel Cobalt Aluminum Oxide (LiNiCoAlO2) — NCA
- Composition: Nickel, cobalt, and aluminum oxide
- Key Traits: Very high energy density, long lifespan, widely used in EVs
- Applications: Tesla EV batteries, industrial energy storage
- Limitations: Expensive, requires robust BMS for safety
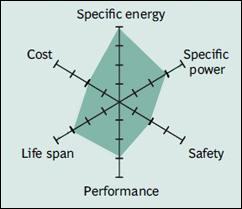 |
| Figure 11: Snapshot of NCA. High energy and power densities, as well as good life span, make NCA a candidate for EV powertrains. High cost and marginal safety are negatives. Source: Cadex |
Summary Table
| Lithium Nickel Cobalt Aluminum Oxide: LiNiCoAlO2 cathode (~9% Co), graphite anode Short form: NCA or Li-aluminum. Since 1999 |
|
| Voltages | 3.60V nominal; typical operating range 3.0–4.2V/cell |
| Specific energy (capacity) | 200-260Wh/kg; 300Wh/kg predictable |
| Charge (C-rate) | 0.7C, charges to 4.20V (most cells), 3h charge typical, fast charge possible with some cells |
| Discharge (C-rate) | 1C typical; 3.00V cut-off; high discharge rate shortens battery life |
| Cycle life | 500 (related to depth of discharge, temperature) |
| Thermal runaway | 150°C (302°F) typical, High charge promotes thermal runaway |
| Cost | ~$350 per kWh (Source: RWTH, Aachen) |
| Applications | Medical devices, industrial, electric powertrain (Tesla) |
| Comments
2019 update: |
Shares similarities with Li-cobalt. Serves as Energy Cell.
Mainly used by Panasonic and Tesla; growth potential. |
Table 12: Characteristics of Lithium Nickel Cobalt Aluminum Oxide.
Lithium Titanate (Li2TiO3) — LTO
- Composition: Lithium titanate anode instead of graphite
- Key Traits: Extremely fast charging, outstanding cycle life (over 10,000 cycles), excellent low-temperature performance
- Applications: Grid storage, military, aerospace, EVs requiring fast charging
- Limitations: Lower energy density, higher cost
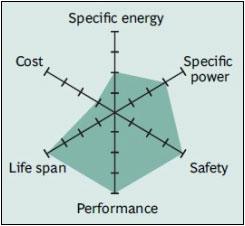 |
| Figure 13: Snapshot of Li-titanate. Li-titanate excels in safety, low-temperature performance and life span. Efforts are being made to improve the specific energy and lower cost. Source: Boston Consulting Group |
Summary Table
| Lithium Titanate: Cathode can be lithium manganese oxide or NMC; Li2TiO3 (titanate) anode Short form: LTO or Li-titanate Commercially available since about 2008. |
|
| Voltages | 2.40V nominal; typical operating range 1.8–2.85V/cell |
| Specific energy (capacity) | 50–80Wh/kg |
| Charge (C-rate) | 1C typical; 5C maximum, charges to 2.85V |
| Discharge (C-rate) | 10C possible, 30C 5s pulse; 1.80V cut-off on LCO/LTO |
| Cycle life | 3,000–7,000 |
| Thermal runaway | One of safest Li-ion batteries |
| Cost | ~$1,005 per kWh (Source: RWTH, Aachen) |
| Applications | UPS, electric powertrain (Mitsubishi i-MiEV, Honda Fit EV), solar-powered street lighting |
| Comments
2019 update: |
Long life, fast charge, wide temperature range but low specific energy and expensive. Among safest Li-ion batteries.
Ability to ultra-fast charge; high cost limits to special application. |
Table 14: Characteristics of lithium titanate.
Future Batteries
Solid-state Li-ion: High specific energy but poor loading and safety.
Lithium-sulfur: High specific energy but poor cycle life and poor loading
Lithium-air: High specific energy but poor loading, needs clean air to breath and has short life.
Figure 15 compares the specific energy of lead-, nickel- and lithium-based systems. While Li-aluminum (NCA) is the clear winner by storing more capacity than other systems, this only applies to specific energy. In terms of specific power and thermal stability, Li-manganese (LMO) and Li-phosphate (LFP) are superior. Li-titanate (LTO) may have low capacity but this chemistry outlives most other batteries in terms of life span and also has the best cold temperature performance. Moving towards the electric powertrain, safety and cycle life will gain dominance over capacity. (LCO stands for Li-cobalt, the original Li-ion.)
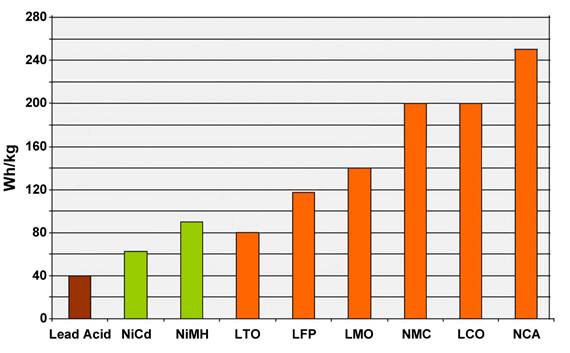
Figure 15: Typical specific energy of lead-, nickel- and lithium-based batteries.
NCA enjoys the highest specific energy; however, manganese and phosphate are superior in terms of specific power and thermal stability. Li-titanate has the best life span.
Courtesy of Cadex